使用预先存在的对照数据在临床前研究中设定期望。
IF 3.5
4区 医学
Q1 MEDICINE, LEGAL
引用次数: 0
摘要
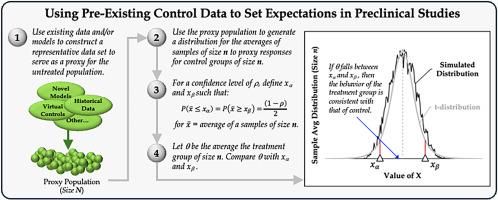
这项工作提出了一种概念性的方法,通过利用已有的对照数据来限制临床前毒理学研究中活体受试者的使用。给定一组有效的预先存在的对照数据,可以在进行研究之前使用概率方法来设定目标研究结果的期望。我们不涉及当前正在进行的生成、模拟、验证或以其他方式建模或构建此类数据集(例如,数学模型、虚拟控制组)的工作。相反,我们假设存在一个适当收集和整理的数据集,这些数据集代表了控制对象的相关指标,并说明了使用概率方法为通常测量的终点的实验结果设定先验期望。我们探索使用t分布来设置小样本量的期望,当端点是连续测量(例如,器官重量),并且数据集的样本平均值是真实总体平均值的良好代理。当端点是离散度量(例如,特定病理的等级)或数据集的样本平均值不能作为一个很好的代理时,我们使用自举来生成分布。我们的结论是,这些概率方法可以帮助研究人员了解未治疗人群中终点的行为,并在询问研究结果时帮助设定对“正常”的先验期望。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Using pre-existing control data to set expectations in preclinical studies
This work presents a conceptual approach to limit the use of live subjects for preclinical toxicological studies by leveraging pre-existing control data. Given a valid set of pre-existing control data, one can use probabilistic methods to set expectations for targeted study outcomes prior to undertaking a study. We do not address current efforts underway to generate, simulate, validate, or otherwise model or construct such data sets (e.g., mathematical models, virtual control groups). Rather, we assume the existence of an appropriately collected and curated data set of relevant metrics representative of control subjects and illustrate the use of probabilistic methods to set expectations a priori for experimental outcomes for commonly measured endpoints. We explore using the T-distribution to set expectations for small sample sizes when endpoints are continuous measures (e.g., organ weights) and the sample average of the data set is a good proxy for the true population mean. When endpoints are discrete measures (e.g., grades of specific pathologies) or the sample average of the data set does not serve as a good proxy, we employ bootstrapping to generate a distribution. We conclude that these probabilistic approaches can help investigators understand the behavior of endpoints in an untreated population and help set a priori expectations for “normalcy” when interrogating study results.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.70
自引率
8.80%
发文量
147
审稿时长
58 days
期刊介绍:
Regulatory Toxicology and Pharmacology publishes peer reviewed articles that involve the generation, evaluation, and interpretation of experimental animal and human data that are of direct importance and relevance for regulatory authorities with respect to toxicological and pharmacological regulations in society. All peer-reviewed articles that are published should be devoted to improve the protection of human health and environment. Reviews and discussions are welcomed that address legal and/or regulatory decisions with respect to risk assessment and management of toxicological and pharmacological compounds on a scientific basis. It addresses an international readership of scientists, risk assessors and managers, and other professionals active in the field of human and environmental health.
Types of peer-reviewed articles published:
-Original research articles of relevance for regulatory aspects covering aspects including, but not limited to:
1.Factors influencing human sensitivity
2.Exposure science related to risk assessment
3.Alternative toxicological test methods
4.Frameworks for evaluation and integration of data in regulatory evaluations
5.Harmonization across regulatory agencies
6.Read-across methods and evaluations
-Contemporary Reviews on policy related Research issues
-Letters to the Editor
-Guest Editorials (by Invitation)

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


