Jagandeep S. Saraya, Derek K. O'Flaherty
{"title":"用氨基修饰剂偶联核酸的水相容柱上方法","authors":"Jagandeep S. Saraya, Derek K. O'Flaherty","doi":"10.1002/cpz1.70169","DOIUrl":null,"url":null,"abstract":"<p>Nucleic acid conjugation has emerged as a powerful strategy for enhancing the chemical and biological versatility of synthetic oligonucleotides. Solid-support synthesis of oligonucleotides provides an avenue for nucleic acid conjugation, so long as the method is inherently compatible with the synthesis cycle. Many methods exist, post-synthetically (i.e., after strand extension via the DNA synthesizer), to add ligands to the strand while it is still bound to the solid support. These, however, tend to require stringent reaction conditions (i.e., anhydrous, degassed solvents, special apparatus), making them sometimes impractical for routine use. This article describes a streamlined aqueous-compatible on-column conjugation strategy for preparing nucleic acids containing site-specific chemical modifications. This method utilizes commercially available or easily synthesized monophosphate or carboxylate-containing ligands and solid-phase synthesized oligonucleotides containing amino-modified termini. Coupling is enabled by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and <i>N</i>-methylimidazole (<i>N</i>-MeIm) chemistry in buffered aqueous-organic mixtures. The resulting conjugates are processed using typical deprotection and solid-support cleavage protocols, purified by standard techniques such as strong anion exchange high-performance liquid chromatography (SAX-HPLC), and are characterized by mass spectrometry (MS). © 2025 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Synthesis of zidovudine 5′-<i>O</i>-monophosphate</p><p><b>Basic Protocol 2</b>: On-column phosphoramidate-mediated conjugation of 5′-<i>O</i>-phosphorylated AZT to 5′-amino DNA</p><p><b>Basic Protocol 3</b>: On-column amide-mediated conjugation of Fmoc-protected glycine to 5′-amino modified DNA</p><p><b>Basic Protocol 4</b>: On-column amide-mediated conjugation of potassium benzoate to 5′-amino modified RNA</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"5 7","pages":""},"PeriodicalIF":2.2000,"publicationDate":"2025-07-04","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.70169","citationCount":"0","resultStr":"{\"title\":\"An Aqueous-Compatible On-Column Approach for the Conjugation of Nucleic Acids Using Amino Modifiers\",\"authors\":\"Jagandeep S. Saraya, Derek K. O'Flaherty\",\"doi\":\"10.1002/cpz1.70169\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Nucleic acid conjugation has emerged as a powerful strategy for enhancing the chemical and biological versatility of synthetic oligonucleotides. Solid-support synthesis of oligonucleotides provides an avenue for nucleic acid conjugation, so long as the method is inherently compatible with the synthesis cycle. Many methods exist, post-synthetically (i.e., after strand extension via the DNA synthesizer), to add ligands to the strand while it is still bound to the solid support. These, however, tend to require stringent reaction conditions (i.e., anhydrous, degassed solvents, special apparatus), making them sometimes impractical for routine use. This article describes a streamlined aqueous-compatible on-column conjugation strategy for preparing nucleic acids containing site-specific chemical modifications. This method utilizes commercially available or easily synthesized monophosphate or carboxylate-containing ligands and solid-phase synthesized oligonucleotides containing amino-modified termini. Coupling is enabled by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and <i>N</i>-methylimidazole (<i>N</i>-MeIm) chemistry in buffered aqueous-organic mixtures. The resulting conjugates are processed using typical deprotection and solid-support cleavage protocols, purified by standard techniques such as strong anion exchange high-performance liquid chromatography (SAX-HPLC), and are characterized by mass spectrometry (MS). © 2025 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Synthesis of zidovudine 5′-<i>O</i>-monophosphate</p><p><b>Basic Protocol 2</b>: On-column phosphoramidate-mediated conjugation of 5′-<i>O</i>-phosphorylated AZT to 5′-amino DNA</p><p><b>Basic Protocol 3</b>: On-column amide-mediated conjugation of Fmoc-protected glycine to 5′-amino modified DNA</p><p><b>Basic Protocol 4</b>: On-column amide-mediated conjugation of potassium benzoate to 5′-amino modified RNA</p>\",\"PeriodicalId\":93970,\"journal\":{\"name\":\"Current protocols\",\"volume\":\"5 7\",\"pages\":\"\"},\"PeriodicalIF\":2.2000,\"publicationDate\":\"2025-07-04\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.70169\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Current protocols\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cpz1.70169\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"\",\"JCRName\":\"\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cpz1.70169","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
An Aqueous-Compatible On-Column Approach for the Conjugation of Nucleic Acids Using Amino Modifiers
Nucleic acid conjugation has emerged as a powerful strategy for enhancing the chemical and biological versatility of synthetic oligonucleotides. Solid-support synthesis of oligonucleotides provides an avenue for nucleic acid conjugation, so long as the method is inherently compatible with the synthesis cycle. Many methods exist, post-synthetically (i.e., after strand extension via the DNA synthesizer), to add ligands to the strand while it is still bound to the solid support. These, however, tend to require stringent reaction conditions (i.e., anhydrous, degassed solvents, special apparatus), making them sometimes impractical for routine use. This article describes a streamlined aqueous-compatible on-column conjugation strategy for preparing nucleic acids containing site-specific chemical modifications. This method utilizes commercially available or easily synthesized monophosphate or carboxylate-containing ligands and solid-phase synthesized oligonucleotides containing amino-modified termini. Coupling is enabled by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-methylimidazole (N-MeIm) chemistry in buffered aqueous-organic mixtures. The resulting conjugates are processed using typical deprotection and solid-support cleavage protocols, purified by standard techniques such as strong anion exchange high-performance liquid chromatography (SAX-HPLC), and are characterized by mass spectrometry (MS). © 2025 The Author(s). Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1: Synthesis of zidovudine 5′-O-monophosphate
Basic Protocol 2: On-column phosphoramidate-mediated conjugation of 5′-O-phosphorylated AZT to 5′-amino DNA
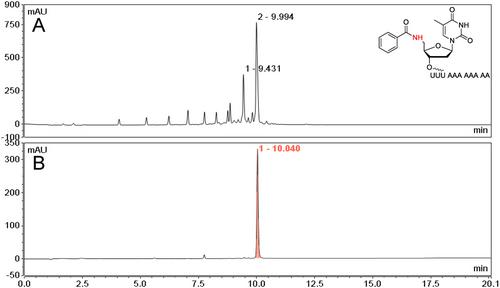
Basic Protocol 3: On-column amide-mediated conjugation of Fmoc-protected glycine to 5′-amino modified DNA
Basic Protocol 4: On-column amide-mediated conjugation of potassium benzoate to 5′-amino modified RNA





 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


