ddensity:使用预训练的深度学习模型嵌入来处理不平衡的药物-药物相互作用风险水平
IF 6.2
2区 医学
Q1 COMPUTER SCIENCE, ARTIFICIAL INTELLIGENCE
引用次数: 0
摘要
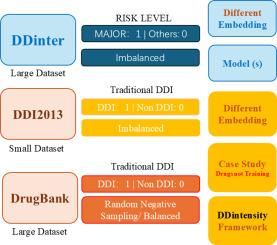
不平衡的数据集一直是生物信息学的一个持续挑战,特别是在药物-药物相互作用(DDI)风险水平数据集的背景下。这种不平衡可能导致有偏见的模型在代表性不足的班级中表现不佳。为了解决这个问题,一种策略是构建一个平衡的数据集,而另一种策略则涉及使用更高级的特征和模型。在本研究中,我们引入了一种名为ddensity的新方法,该方法利用预训练的深度学习模型作为嵌入生成器,结合lstm -注意力模型来解决DDI风险水平数据集的不平衡问题。我们测试了来自不同领域的嵌入,包括图像、图形和文本语料库。其中,由BioGPT生成的嵌入具有最高的性能,曲线下面积(AUC)为0.97,Precision-Recall曲线下面积(AUPR)为0.92。我们的模型在DDinter上进行了训练,并使用MecDDI数据集进一步验证。此外,还对用于肿瘤的化疗药物DB00398(索拉非尼)和DB01204(米托蒽醌)进行了案例研究,以证明该方法的特异性和有效性。我们的方法展示了跨DDI模式的高可扩展性,以及新交互的发现。总之,我们引入ddinsity作为生物信息学中使用预训练深度学习嵌入的不平衡数据集的解决方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
DDintensity: Addressing imbalanced drug-drug interaction risk levels using pre-trained deep learning model embeddings
Imbalanced datasets have been a persistent challenge in bioinformatics, particularly in the context of drug-drug interaction (DDI) risk level datasets. Such imbalance can lead to biased models that perform poorly on underrepresented classes. To address this issue, one strategy is to construct a balanced dataset, while another involves employing more advanced features and models. In this study, we introduce a novel approach called DDintensity, which leverages pre-trained deep learning models as embedding generators combined with LSTM-attention models to address the imbalance in DDI risk level datasets. We tested embeddings from various domains, including images, graphs, and textual corpus. Among these, embeddings generated by BioGPT achieved the highest performance, with an Area Under the Curve (AUC) of 0.97 and an Area Under the Precision-Recall curve (AUPR) of 0.92. Our model was trained on the DDinter and further validated using the MecDDI dataset. Additionally, case studies on chemotherapeutic drugs, DB00398 (Sorafenib) and DB01204 (Mitoxantrone) used in oncology, were conducted to demonstrate the specificity and effectiveness of the this methods. Our approach demonstrates high scalability across DDI modalities, as well as the discovery of novel interactions. In summary, we introduce DDIntensity as a solution for imbalanced datasets in bioinformatics with pre-trained deep-learning embeddings.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Artificial Intelligence in Medicine
工程技术-工程:生物医学
CiteScore
15.00
自引率
2.70%
发文量
143
审稿时长
6.3 months
期刊介绍:
Artificial Intelligence in Medicine publishes original articles from a wide variety of interdisciplinary perspectives concerning the theory and practice of artificial intelligence (AI) in medicine, medically-oriented human biology, and health care.
Artificial intelligence in medicine may be characterized as the scientific discipline pertaining to research studies, projects, and applications that aim at supporting decision-based medical tasks through knowledge- and/or data-intensive computer-based solutions that ultimately support and improve the performance of a human care provider.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


