等离子活化水中活性氧和活性氮降解黄曲霉毒素B1的DFT研究
IF 3
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
在M06-2X/aug-cc-pVTZ//M06-2X/6-31G(d)水平上对等离子活化水中活性氧和活性氮对黄曲霉毒素B1的降解机理进行了理论研究。所有研究反应的扩散速率系数计算为~ 109 M−1 s−1,相关活化能约为18-19 kJ mol−1。最有利的降解途径是添加末端呋喃环的乙烯键。298.15 K时,•OH、臭氧、•O2−、•NO和NO3−触发的表观速率系数分别为109、104、10−8、10−7和10−41 M−1 s−1,说明•OH和臭氧在降解过程中起有效作用,而•O2−、•NO和NO3−的影响可以忽略。•OH和臭氧在末端呋喃环乙烯基键上加成的表观速率系数分别为17.02和18.96 kJ mol−1,相应的支化率分别为82.8%和86.28%。利用定量构效关系方法对主要降解加合物的生态毒性和诱变性进行了评价。黄曲霉毒素B1作为诱变化合物对蚯蚓和水生生物无急性和慢性毒性,但对大鼠有毒性。最丰富的氧化产物的致突变性为负,毒性低于AFB1。本文章由计算机程序翻译,如有差异,请以英文原文为准。
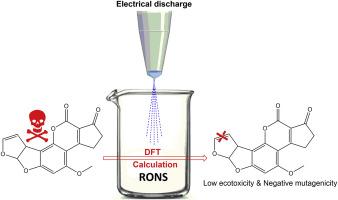
A DFT study on degradation of aflatoxin B1 using some reactive oxygen and nitrogen species of plasma-activated water
The degradation mechanism of aflatoxin B1 using some reactive oxygen and nitrogen species of plasma-activated water have been investigated theoretically at the M06-2X/aug-cc-pVTZ//M06-2X/6-31G(d) level. Diffusion rate coefficient for all studied reactions was calculated to be ∼109 M−1 s−1 and related activation energy was about 18–19 kJ mol−1. The most favorable pathways of degradation were addition the vinyl bond of terminal furan ring. The apparent rate coefficients triggered by •OH, ozone, •O2−, •NO, and NO3− at 298.15 K were 109, 104, 10−8, 10−7, and 10−41 M−1 s−1, implying that •OH and ozone play efficient role in the degradation while the effect of •O2−, •NO, and NO3− species can be ignored. Overall activation energies associated with the apparent rate coefficients for addition of •OH and ozone to the vinyl bond of terminal furan ring were 17.02 and 18.96 kJ mol−1, while corresponding branching ratios were 82.8 % and 86.28 %, respectively. Ecotoxicity and mutagenicity of major degradation adducts have been estimated using the quantitative structure activity relationships methodologies. Aflatoxin B1 as mutagenic compound does not show acute and chronic toxicity to earthworm and aquatic organisms but was toxic for rats. Mutagenicity of the most abundant oxidation products was negative while the toxicity of them was less than AFB1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of molecular graphics & modelling
生物-计算机:跨学科应用
CiteScore
5.50
自引率
6.90%
发文量
216
审稿时长
35 days
期刊介绍:
The Journal of Molecular Graphics and Modelling is devoted to the publication of papers on the uses of computers in theoretical investigations of molecular structure, function, interaction, and design. The scope of the journal includes all aspects of molecular modeling and computational chemistry, including, for instance, the study of molecular shape and properties, molecular simulations, protein and polymer engineering, drug design, materials design, structure-activity and structure-property relationships, database mining, and compound library design.
As a primary research journal, JMGM seeks to bring new knowledge to the attention of our readers. As such, submissions to the journal need to not only report results, but must draw conclusions and explore implications of the work presented. Authors are strongly encouraged to bear this in mind when preparing manuscripts. Routine applications of standard modelling approaches, providing only very limited new scientific insight, will not meet our criteria for publication. Reproducibility of reported calculations is an important issue. Wherever possible, we urge authors to enhance their papers with Supplementary Data, for example, in QSAR studies machine-readable versions of molecular datasets or in the development of new force-field parameters versions of the topology and force field parameter files. Routine applications of existing methods that do not lead to genuinely new insight will not be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


