在系统评价和荟萃分析中,随机对照试验的个体参与者数据为偏倚风险评估提供了信息。
IF 5.2
2区 医学
Q1 HEALTH CARE SCIENCES & SERVICES
引用次数: 0
摘要
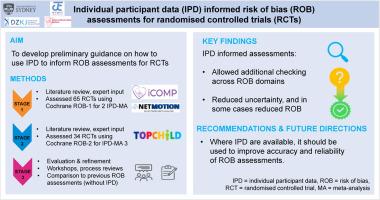
目的:在证据合成中,评估合格研究的偏倚风险(ROB)对于解释研究结果至关重要。像Cochrane的rob1或rob2这样的标准化工具传统上依赖于公布的信息来进行评估,但这通常是不完整或不明确的。原始个人参与者数据(IPD)的可用性使更深入的评估成为可能,然而,缺乏关于如何在ROB评估中使用IPD的指导。我们的目标是就如何使用IPD为三个案例研究的随机对照试验(rct)的ROB评估提供初步指导。研究设计和设置:在第一阶段,我们回顾了相关文献,咨询了我们的网络,并借鉴了以往的经验,编制了关于IPD如何为每个领域的ROB评估提供信息的项目。我们与国际跨学科专家咨询小组讨论了每个项目的可行性和潜在用途,并制定了初步指南,并在使用rob1的两项IPD荟萃分析(MAs)(65项随机对照试验)中进行了试点。在第2阶段,该指南适用于rob2,并应用于另一个IPD-MA(34个rct)。所有评估均由两名独立审查人员进行,一式两份。在第3阶段,我们进行了一次评估研讨会,以进一步细化每个项目,并获得重要的经验教训。为了评估ipd评估的影响,我们将它们与33项试验中仅使用已发表信息进行的现有rob1评估进行了比较。结果:我们在ROB域中鉴定出12个项目。IPD通过对选择偏差(即测试随机化)和损耗偏差(即在不同时间点对不完整数据进行更细粒度的评估)进行额外检查,为加强ROB评估提供了机会。我们还确定了IPD的可用性可以减少ROB的领域,例如,通过减轻选择性结果报告偏倚或通过在意向治疗分析中重新纳入被排除的参与者。在33项研究中,应用ipd评估导致25项研究中的ROB判断发生变化,最常见的是,以前标记为“不清楚”的域的解决。结论:我们对ipd知情的ROB评估的初步指导可应用于IPD-MAs,以提高ROB评估的准确性,并在某些情况下减少ROB,以创建更可靠的证据基础,为政策和实践提供信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Individual participant data–informed risk of bias assessments for randomized controlled trials in systematic reviews and meta-analyses
Objectives
In evidence synthesis, assessing risk of bias (ROB) of eligible studies is crucial to inform interpretation of findings. Standardized tools like Cochrane's ROB-1 or ROB-2 traditionally rely on published information to inform assessments, but this is often incomplete or unclear. Availability of raw individual participant data (IPD) enables more in-depth assessments; however, guidance on how to use IPD in ROB assessments is lacking. We aim to develop preliminary guidance on how to use IPD to inform ROB assessments of randomized controlled trials (RCTs) for three case studies.
Study Design and Setting
In stage 1, we reviewed relevant literature, consulted our networks, and drew on previous experience to compile items on how IPD may inform ROB assessment for each domain. We discussed feasibility and potential usefulness of each item with an international, interdisciplinary expert advisory group and developed preliminary guidance, which was piloted in two IPD meta-analyses (MAs) (65 RCTs) using ROB-1. In stage 2, the guide was adapted for ROB-2 and applied to another IPD-MA (34 RCTs). All assessments were conducted in duplicate by two independent reviewers. In stage 3, we conducted an evaluation workshop to further refine each item, and capture important lessons. To assess the impact of IPD-informed assessments, we compared them to existing ROB-1 assessments performed with published information alone for 33 trials.
Results
We identified 12 items across the ROB domains. IPD provided opportunities to enhance ROB assessments by enabling additional checks for selection bias (ie, testing randomization) and attrition bias (ie, more granular assessment of incomplete data at various time points). We also identified domains for which availability of IPD enabled reduction of ROB, for instance, by mitigating selective outcome reporting bias or by reincluding excluded participants in intention-to-treat analyses. Applying IPD-informed assessments led to changes in ROB judgment in 25 of 33 studies, most commonly, resolution of domains previously marked as “unclear”.
Conclusion
Our preliminary guidance for IPD-informed ROB assessments may be applied in IPD-MAs to increase the accuracy of ROB assessments and in some cases reduce ROB to create a more reliable evidence base informing policy and practice.
Plain Language Summary
When making decisions about how to treat a patient in clinical practice, it is important to consider the results of all relevant studies. Usually, combined analyses of multiple clinical trials rely on published reports, in which researchers summarize their findings. However, looking at the original data from these studies, instead of just the published reports, can improve the quality of analyses. Access to these underlying data also allows for more thorough assessment of the studies' quality and any potential for bias. This is important for understanding the results properly and for making the most appropriate treatment decisions for patients. Here, we present guidance on how to assess risk of bias of trials using these original datasets.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Clinical Epidemiology
医学-公共卫生、环境卫生与职业卫生
CiteScore
12.00
自引率
6.90%
发文量
320
审稿时长
44 days
期刊介绍:
The Journal of Clinical Epidemiology strives to enhance the quality of clinical and patient-oriented healthcare research by advancing and applying innovative methods in conducting, presenting, synthesizing, disseminating, and translating research results into optimal clinical practice. Special emphasis is placed on training new generations of scientists and clinical practice leaders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


