usp7介导的NUF2去泛素化通过调节SLC7A11表达加速卵巢癌进展。
IF 3.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
摘要
背景:卵巢癌是一种高致死率的恶性肿瘤。以往的研究表明Ndc80着丝粒复合物组分(NUF2)在卵巢癌中表现出致癌特性。本研究旨在探讨NUF2促进卵巢癌进展的潜在机制。方法:采用逆转录定量聚合酶链反应(RT-qPCR)检测NUF2及溶质载体家族7成员11 (SLC7A11) mRNA表达水平。Western blot检测NUF2、泛素特异性蛋白酶7 (USP7)和SLC7A11蛋白的表达。采用3-(4,5-二甲基噻唑-2-基)-2,5-二苯基-2 h -溴化四唑(MTT)和5-乙基-2'-脱氧尿苷(EdU)测定细胞增殖。流式细胞术检测细胞凋亡,transwell侵袭实验检测细胞侵袭。通过检测铁离子(Fe2+)、活性氧(ROS)和丙二醛(MDA)水平来评估铁下垂。免疫沉淀(IP)和泛素化实验证实了NUF2和USP7之间的关系。在体内,使用异种移植小鼠模型检测NUF2在卵巢癌中的作用,然后进行免疫组织化学(IHC)分析。结果:NUF2在卵巢癌细胞中显著升高。敲低NUF2可显著抑制卵巢癌细胞的增殖和侵袭,促进细胞凋亡和铁下垂。在机制上,USP7通过介导其去泛素化来稳定NUF2。USP7缺失抑制卵巢癌细胞增殖和侵袭,促进细胞凋亡和铁凋亡,这些作用是通过下调NUF2介导的。此外,SLC7A11在卵巢癌中过表达,NUF2正调控其表达。NUF2缺失降低了SLC7A11的表达,从而抑制了卵巢癌的进展。此外,SLC7A11过表达可以逆转USP7敲低对卵巢癌进展的抑制作用。结论:USP7通过去泛素化作用稳定NUF2表达,通过调节SLC7A11表达加速卵巢癌进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
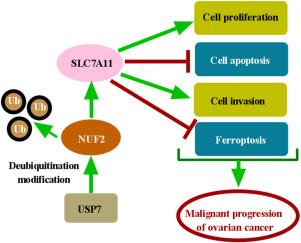
USP7-mediated NUF2 deubiquitination accelerates ovarian cancer progression through regulating SLC7A11 expression
Background
Ovarian cancer is highly lethal malignancy. Previous studies have indicated that Ndc80 kinetochore complex component (NUF2) exhibited oncogenic properties in ovarian cancer. This study aimed to investigate the underlying mechanisms by which NUF2 contributes to ovarian cancer progression.
Methods
Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was conducted to measure the mRNA expression levels of NUF2 and solute carrier family 7 member 11 (SLC7A11). The protein expression of NUF2, ubiquitin-specific protease 7 (USP7) and SLC7A11 was examined through Western blot. Cell proliferation was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2 H-tetrazolium bromide (MTT) and 5-ethynyl-2′-deoxyuridine (EdU) assays. Cell apoptosis and invasion were detected by flow cytometry and transwell invasion assay, respectively. Ferroptosis was assessed through detecting ferrous ion (Fe2+), reactive oxygen species (ROS) and malondialdehyde (MDA) levels. Immunoprecipitation (IP) and ubiquitination assays were conducted to prove the relationship between NUF2 and USP7. In vivo, the role of NUF2 in ovarian cancer was examined using xenograft mouse models, followed by immunohistochemistry (IHC) analysis.
Results
NUF2 was significantly elevated in ovarian cancer cells. Knockdown of NUF2 markedly inhibited ovarian cancer cell proliferation and invasion while promoting apoptosis and ferroptosis. Mechanistically, USP7 stabilized NUF2 by mediating its deubiquitination. USP7 depletion inhibited cell proliferation and invasion, and promoted cell apoptosis and ferroptosis in ovarian cancer cells, effects that were mediated through NUF2 downregulation. Furthermore, SLC7A11 was overexpressed in ovarian cancer, and NUF2 positively regulated its expression. NUF2 depletion decreased SLC7A11 expression, thereby suppressing ovarian cancer progression. Additionally, SLC7A11 overexpression could reverse the inhibitory effects of USP7 knockdown on ovarian cancer progression.
Conclusion
USP7 stabilized NUF2 expression via deubiquitination to accelerate ovarian cancer progression through regulating SLC7A11 expression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


