增强子劫持驱动FAM20C表达促进甲状腺乳头状癌进展。
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
甲状腺乳头状癌(PTC)是最常见的内分泌肿瘤,多数预后良好。然而,侵袭性PTC可转移或复发,成为难治性疾病。因此,迫切需要发现新的侵袭性PTC生物标志物。越来越多的证据表明,异常增强子和靶向基因转录驱动PTC的进展。为了鉴定PTC中的癌症特异性增强子及其下游基因,我们分析了3例PTC患者的癌症组织和匹配的正常组织的转录组(RNA-seq)和基于增强子的表观基因组重组(ChIP-seq)。重要的是,六个候选基因(RHBDF1、FAM20C、PHLDA2、TMPRSS6、LAD1和BGN)在PTC中被增强子持续上调,并与预后相关。进一步的实验验证了FAM20C增强子调控PTC肿瘤发生的功能,从而揭示了FAM20C调控的PTC两种细胞因子(TNF-α和TGF-β)的抑癌机制。此外,我们在体外和体内证明了FAM20C抑制剂(3r)抑制甲状腺癌细胞的增殖和侵袭。此外,FAM20C是由KLF12通过其增强子驱动的。总的来说,我们的研究揭示了癌症特异性增强子的异常激活与PTC肿瘤发生之间的潜在相关性,并确定了FAM20C作为PTC的新靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
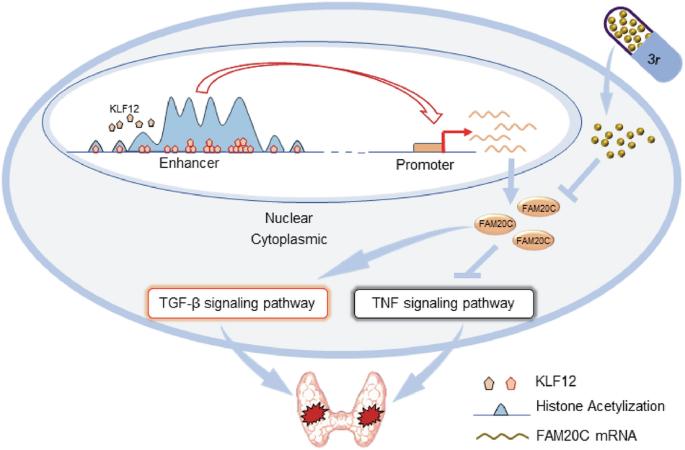
Enhancer hijacking drives FAM20C expression to promote papillary thyroid cancer progression
Papillary thyroid cancer (PTC) is the most common endocrine cancer, with a good prognosis in most cases. However, aggressive PTC can metastasise or reoccur and become refractory disease. Therefore, it’s urgent to uncover new biomarkers for aggressive PTC. Accumulating evidence suggests that aberrant enhancers and targeted gene transcription drive the progression of PTC. To identify the cancer-specific enhancers and their downstream genes in PTC, we profiled the transcriptomes (RNA-seq) and enhancer-based epigenomic reorganisation (ChIP-seq) of cancer tissues and matched normal tissues from three PTC patients. Importantly, six candidate genes (RHBDF1, FAM20C, PHLDA2, TMPRSS6, LAD1, and BGN) were identified to be consistently upregulated by enhancers in PTC and correlated with prognosis. Further experiments verified the function of enhancers governing FAM20C in regulating PTC tumorigenesis, thereby unveiling a FAM20C-governed oncogenic mechanism for suppressing two cytokines (TNF-α and TGF-β) in PTC. Additionally, we demonstrated that a FAM20C inhibitor (3r) suppressed the proliferation and invasion of thyroid cancer cells in vitro and vivo. Moreover, FAM20C is driven by KLF12 through its enhancer. Collectively, our study uncovers the potential correlations between the aberrant activation of cancer-specific enhancers and PTC tumorigenesis and identifies FAM20C as a novel target for PTC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


