大型语言模型,加速有机化学合成
IF 23.9
1区 计算机科学
Q1 COMPUTER SCIENCE, ARTIFICIAL INTELLIGENCE
引用次数: 0
摘要
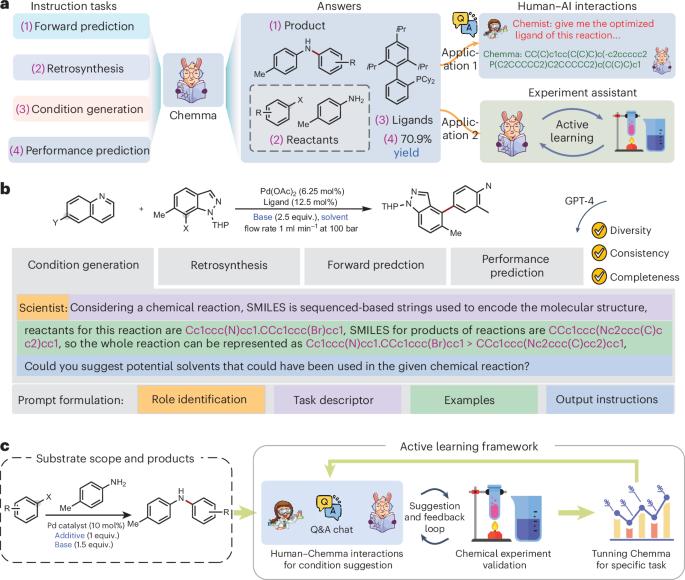
化学合成作为创造变革性分子的基础方法,在从生命科学到材料和能源的各个领域都具有重大影响。目前的化学合成实践强调了费力和昂贵的试错工作流程,强调了对先进人工智能助手的迫切需求。近年来,以GPT-4为代表的大型语言模型作为促进科学研究的有效工具被引入。在这里,我们提出Chemma,一个完全微调的大型语言模型,有128万对关于反应的问题和答案,作为加速有机化学合成的助手。Chemma在多个化学任务中超越了最著名的结果,例如单步反合成和产量预测,这凸显了通用人工智能在有机化学中的潜力。通过预测整个实验反应空间的产率,Chemma显著提高了贝叶斯优化的反应勘探能力。更重要的是,Chemma集成在主动学习框架中,在开放的反应空间中展示了自主实验探索和优化的先进潜力。对于未报道的环氨基硼酸盐和芳基卤化物的Suzuki-Miyaura交叉偶联反应,人工智能合作仅用15次就成功地找到了合适的配体(三(1-金刚烷)膦)和溶剂(1,4-二恶烷),分离收率达到67%。这些结果表明,在没有量子化学计算的情况下,Chemma可以以类似于人类专家的方式从反应数据中理解和提取化学见解。这项工作为加速有机化学合成与适应的大语言模型开辟了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Large language models to accelerate organic chemistry synthesis
Chemical synthesis, as a foundational methodology in the creation of transformative molecules, exerts substantial influence across diverse sectors from life sciences to materials and energy. Current chemical synthesis practices emphasize laborious and costly trial-and-error workflows, underscoring the urgent needs for advanced AI assistants. Recently, large language models, typified by GPT-4, have been introduced as an efficient tool to facilitate scientific research. Here we present Chemma, a fully fine-tuned large language model with 1.28 million pairs of questions and answers about reactions, as an assistant to accelerate organic chemistry synthesis. Chemma surpasses the best-known results in multiple chemical tasks, for example, single-step retrosynthesis and yield prediction, which highlights the potential of general artificial intelligence for organic chemistry. By predicting yields across the experimental reaction space, Chemma significantly improves the reaction exploration capability of Bayesian optimization. More importantly, integrated in an active learning framework, Chemma exhibits advanced potentials of autonomously experimental exploration and optimization in open reaction spaces. For an unreported Suzuki–Miyaura cross-coupling reaction of cyclic aminoboronates and aryl halides for the synthesis of α-aryl N-heterocycles, the human–artificial intelligence collaboration successfully explored a suitable ligand (tri(1-adamantyl)phosphine) and solvent (1,4-dioxane) within only 15 runs, achieving an isolated yield of 67%. These results reveal that, without quantum-chemical calculations, Chemma can comprehend and extract chemical insights from reaction data, in a manner akin to human experts. This work opens avenues for accelerating organic chemistry synthesis with adapted large language models. Large language models (LLMs) can be useful tools for science, but they often lack expert understanding of complex domains that they were not trained on. Zhang and colleagues fine-tuned a LLaMA-2-7b-based LLM with questions on organic chemistry reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Machine Intelligence
Multiple-
CiteScore
36.90
自引率
2.10%
发文量
127
期刊介绍:
Nature Machine Intelligence is a distinguished publication that presents original research and reviews on various topics in machine learning, robotics, and AI. Our focus extends beyond these fields, exploring their profound impact on other scientific disciplines, as well as societal and industrial aspects. We recognize limitless possibilities wherein machine intelligence can augment human capabilities and knowledge in domains like scientific exploration, healthcare, medical diagnostics, and the creation of safe and sustainable cities, transportation, and agriculture. Simultaneously, we acknowledge the emergence of ethical, social, and legal concerns due to the rapid pace of advancements.
To foster interdisciplinary discussions on these far-reaching implications, Nature Machine Intelligence serves as a platform for dialogue facilitated through Comments, News Features, News & Views articles, and Correspondence. Our goal is to encourage a comprehensive examination of these subjects.
Similar to all Nature-branded journals, Nature Machine Intelligence operates under the guidance of a team of skilled editors. We adhere to a fair and rigorous peer-review process, ensuring high standards of copy-editing and production, swift publication, and editorial independence.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


