从螯合物到合理设计的五齿配体负载的无膦Co(iii)催化剂用于醇的活化:9h -芴的sp3 C-H烷基化和喹啉合成的研究
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究引入了一种五齿羧基酰胺配体(BPAPA- h =(2-(双(吡啶-2-甲基)氨基)- n ' -苯基- n ' -(吡啶-2-基)乙酰肼),并利用该配体合成了钴(III)催化剂[Co(III)(BPAPA)Cl]ClO4 (C1)和[Co(III)(BPAPA)Br]ClO4 (C2)。这些钴(III)催化剂通过借氢(BH)方法与廉价而丰富的醇进行9h -芴选择性单sp3 C-H烷基化反应,并产生生态良性水作为副产物。采用现有的方法,以不同的芳香族和脂肪族醇为烷基化剂,制备了41个单sp3 C-H烷基化芴衍生物,分离收率高达97%。为了考察其在杂环化合物合成中的催化潜力,我们优化的钴(III)催化剂促进了2-氨基苄醇和芳香酮的无受体脱氢偶联,生成了25个喹啉衍生物,收率高达96%。目前的方法还在克级合成sp3 C-H烷基化芴和喹啉的大规模应用中进行了探索。为了解释和揭示可能的反应机理和中间体,进行了一系列的对照实验。利用HRMS对催化循环中涉及的关键中间体进行了表征。本文章由计算机程序翻译,如有差异,请以英文原文为准。
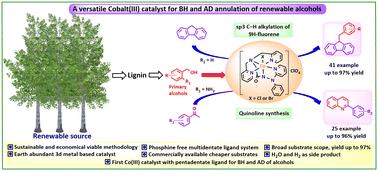
A passage from pincer complexes to rationally designed phosphine-free Co(iii) catalysts supported by a pentadentate ligand for activation of alcohols: studies on sp3 C–H alkylation of 9H-fluorene and quinoline synthesis†
In this study, a pentadentate carboxamide ligand (BPAPA-H = (2-(bis(pyridin-2-ylmethyl)amino)-N′-phenyl-N′-(pyridin-2-yl)acetohydrazide) is introduced and utilized for the synthesis of cobalt(iii) catalysts [Co(iii)(BPAPA)Cl]ClO4 () and [Co(iii)(BPAPA)Br]ClO4 (). These cobalt(iii) catalysts are used for selective mono sp3 C–H alkylation of 9H-fluorene with cheap and abundant alcohols via the borrowing hydrogen (BH) approach, with the generation of ecologically benign water as a side product. Employing the existing methodology, 41 derivatives of mono sp3 C–H alkylated fluorene were produced utilizing different aromatic and aliphatic alcohols as alkylating agents, with isolated yields reaching as high as 97%. To investigate the catalytic potential in the synthesis of heterocycles, our optimized cobalt(iii) catalyst facilitated the acceptorless dehydrogenative (AD) coupling of 2-aminobenzyl alcohol with aromatic ketones, resulting in the formation of 25 quinoline derivatives with yields reaching as high as 96%. The current methodology was also explored in the gram-scale synthesis of sp3 C–H alkylation of fluorene and quinoline synthesis for large-scale applications. A series of control experiments were carried out to explain and reveal the possible reaction mechanism and intermediates. The key intermediates involved in the catalytic cycle were characterized with the help of HRMS studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


