修饰hTERT启动子驱动的嘌呤核苷磷酸化酶基因治疗与卵巢癌化疗和靶向治疗相关
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
卵巢癌是全世界妇科癌症中死亡率与发病率最高的癌症。以大肠杆菌嘌呤核苷磷酸化酶为基础的基因导向酶前药治疗(PNP-GDEPT)为实体瘤的治疗提供了一种有前景的替代方案。本研究通过使用最近开发的两种修饰的hTERT启动子:突变的hTERT (hTERTm)和嵌合的hTERT- cmv,提出了一种原始的卵巢癌治疗方法。我们设计了四个质粒来研究癌症特异性PNP基因表达的影响:pCMV-PNP、phTERT-PNP、phTERTm-PNP和phTERT-CMV-PNP。采用阳离子脂质制剂BSV163/DOPE将pnp编码质粒转染顺铂敏感卵巢癌细胞和耐药卵巢癌细胞。htert驱动的PNP-GDEPT选择性地降低了癌细胞的活力,同时保留了原代人成纤维细胞。此外,PNP-GDEPT联合顺铂或奥拉帕尼进一步增强了对细胞活力和凋亡的抗癌作用。然而,同时使用顺铂和奥拉帕尼,无论是否使用PNP-GDEPT,均未观察到联合效应。我们的研究结果表明,靶向PNP-GDEPT有可能提高化疗和靶向治疗卵巢癌的疗效,同时最大限度地减少对健康细胞的副作用。无论顺铂耐药与否,这种治疗都是有效的,值得进一步研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
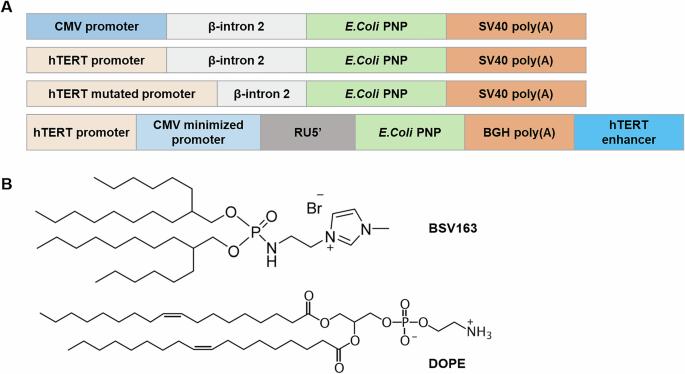
Modified hTERT promoters-driven purine nucleoside phosphorylase-gene therapy in association with chemo- and targeted therapy in the context of ovarian cancer
Ovarian cancer has the highest mortality-to-incidence ratio among gynecologic cancers worldwide. E.coli Purine Nucleoside Phosphorylase-based gene-directed enzyme prodrug therapy (PNP-GDEPT) offers a promising alternative for the treatment of solid tumors. This study proposes an original ovarian cancer treatment through the use of two recently developed modified hTERT promoters: the mutated hTERT (hTERTm) and the chimeric hTERT-CMV. Four plasmids were engineered to investigate the effects of cancer-specific PNP gene expression: pCMV-PNP, phTERT-PNP, phTERTm-PNP, and phTERT-CMV-PNP. The cationic lipid formulation BSV163/DOPE was employed to transfect PNP-coding plasmids into cisplatin-sensitive ovarian cancer cells and their resistant counterparts. hTERT-driven PNP-GDEPT selectively reduced cancer cell viability while sparing primary human fibroblasts. In addition, combining PNP-GDEPT with either cisplatin or olaparib further enhanced anticancer effects on cell viability and apoptosis. However, no combined effects were observed for the concurrent use of cisplatin and olaparib, with or without PNP-GDEPT. Our results demonstrate that targeted PNP-GDEPT has the potential to enhance the efficacy of chemotherapy and targeted therapy against ovarian cancer while minimizing side effects on healthy cells. This treatment is effective irrespective of cisplatin resistance status and warrants further investigation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


