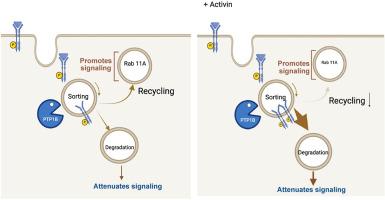
激活素A通过干扰活化的VEGFR2的运输来防止病理血管中对VEGF的高反应性。
IF 3.6
2区 医学
Q1 PATHOLOGY
引用次数: 0
摘要
虽然糖尿病视网膜病变(DR)等影响生活质量的疾病是由血管内皮生长因子(VEGF)驱动的,但越来越多的人认识到,VEGF的浓度并不是视网膜内血管功能障碍的唯一影响因素。激活素A (Activin A,激活素)是转化生长因子β超家族(TGF-β)的配体,可减弱vegf诱导的VE-cadherin的破坏、孔形成和原代人视网膜内皮细胞(HRECs)的通透性。进一步研究这一现象的机制发现,激活素降低了Rab11的表达,这是激活素发挥作用所必需的。在胞内定位蛋白酪氨酸磷酸酶(PTP1b)表达被抑制的细胞中未观察到激活素的作用,而存在于质膜上的PTPs则是可有可无的。激活素在刺激后30分钟及更长的时间点减弱VEGF介导的VEGF受体2 (VEGFR2)磷酸化。总之,这些数据支持激活素通过干扰激活的VEGFR2的运输来抑制vegf诱导的屏障松弛的概念。这种基于激活素的对VEGF反应性的抑制在终末期增殖性糖尿病视网膜病变(PDR)患者的病理血管内皮中受到损害。这一缺陷使得HRECs对VEGF反应过度,并且在糖尿病小鼠的视网膜血管中未观察到,糖尿病小鼠不会发展成血管生成形式的dr。这些数据为患者PDR的发病机制提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Activin A Prevents Hyperresponsiveness to Vascular Endothelial Growth Factor in Pathologic Blood Vessels by Perturbing the Trafficking of Activated Vascular Endothelial Growth Factor Receptor 2
Although quality-of-life compromising afflictions, such as diabetic retinopathy (DR), are driven by vascular endothelial growth factor (VEGF), there is a growing appreciation that the concentration of VEGF is not the only influencer of vascular dysfunction within the retina. Herein, activin A (activin), a ligand of the transforming growth factor-β superfamily, attenuated VEGF-induced vascular endothelial–cadherin disorganization, pore formation, and permeability of primary human retinal endothelial cells. Efforts to further investigate the mechanism of this phenomenon revealed that activin reduced the expression of Rab11, which was required for the activin effect. The activin effect was not observed in cells with suppressed expression of the endosomally localized protein tyrosine phosphatase (PTP1b), whereas protein tyrosine phosphatases present on the plasma membrane were dispensable. Activin attenuated VEGF-mediated phosphorylation of VEGF receptor 2 at 30 minutes and longer post-stimulation time points. Together, these data support the concept that activin suppressed VEGF-induced barrier relaxation by perturbing trafficking of activated VEGF receptor 2. This activin-based suppression of responsiveness to VEGF was compromised in the endothelium of pathologic blood vessels from patients who developed end-stage proliferative DR. This defect rendered human retinal endothelial cell hyperresponsive to VEGF and was not observed in retinal vessels of diabetic mice, which do not develop the angiogenic forms of DR. These data provide novel insights into the pathogenesis of proliferative DR in patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


