钯催化串联C-H活化色氨酸衍生物的一锅c2 -芳基化和c4 -乙酰氧基化
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们揭示了通过钯催化的级联C-H活化在吲哚环的C2和C4位置的色氨酸衍生物的双功能化。这个步骤经济的方案在温和的条件下操作简单,在一个锅中同时实现芳基和乙氧基的安装,使其成为一种直接有效地合成高修饰色氨酸衍生物的方法。此外,克尺度合成和进一步变换也是可行的,证明了该方法的鲁棒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
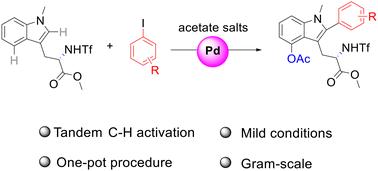
One-pot C2-arylation and C4-acetoxylation of tryptophan derivatives via palladium-catalyzed tandem C–H activation
Herein, we disclosed a dual-functionalization of tryptophan derivatives at the C2 and C4 positions of the indole ring through a palladium-catalyzed cascade C–H activation. This step-economical protocol features operational simplicity under mild conditions, achieving concurrent aryl and acetoxy group installation in one pot, making it a straightforward approach to efficiently synthesize highly decorated tryptophan derivatives. Furthermore, gram-scale synthesis and further transformation were also feasible, demonstrating the robustness of this method.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


