肾可清除的超小单晶铁纳米颗粒用于高选择性和有效的铁下垂治疗和免疫治疗
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 85
摘要
铁基纳米颗粒因其通过催化芬顿反应诱导铁下垂和进一步增强免疫治疗的能力而受到广泛关注。然而,目前的铁基纳米颗粒由于其产生活性氧的效率较低,需要与其他治疗方法合作使用或大剂量应用才能有效治疗。在这里,我们合成了超小单晶铁纳米粒子(bcc-USINPs),它在正常生理环境中保持稳定,但在肿瘤微环境中却高度活跃,因为Fe3O4外壳的选择性酸性蚀刻和Fe(0)核心的暴露。bcc-USINPs在极低浓度下可有效诱导肿瘤细胞铁下垂和免疫遗传细胞死亡。静脉注射iRGD-bcc-USINPs 3次剂量均为1 mg/kg,可有效抑制肿瘤生长,促进树突状细胞成熟,引发适应性T细胞反应。结合程序性死亡配体1 (PD-L1)免疫检查点阻断免疫疗法,irgd -bcc- usinp介导的铁凋亡疗法极大地增强了免疫应答,并形成了强大的免疫记忆。此外,这些USINPs在正常组织中可以快速从肾脏排出,没有副作用。这些iRGD-bcc-USINPs为基于铁中毒的免疫治疗提供了一种简单、安全、有效和选择性肿瘤反应的铁(0)递送系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。
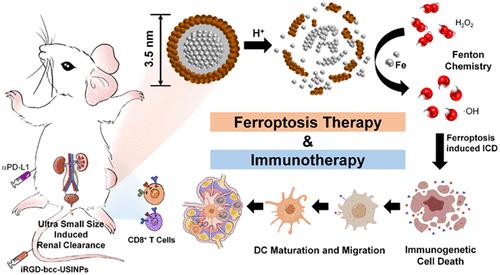
Renal Clearable Ultrasmall Single-Crystal Fe Nanoparticles for Highly Selective and Effective Ferroptosis Therapy and Immunotherapy
Iron-based nanoparticles have attracted much attention because of their ability to induce ferroptosis via a catalyzing Fenton reaction and to further potentiate immunotherapy. However, current iron-based nanoparticles need to be used in cooperation with other treatments or be applied in a high dose for effective therapy because of their low reactive oxygen species production efficacy. Here, we synthesized ultrasmall single-crystal Fe nanoparticles (bcc-USINPs) that stayed stable in a normal physiological environment but were highly active in a tumor microenvironment because of the selective acidic etching of an Fe3O4 shell and the exposure of the Fe(0) core. The bcc-USINPs could efficiently induce tumor cell ferroptosis and immunogenetic cell death at a very low concentration. Intravenous injection of iRGD-bcc-USINPs at three doses of 1 mg/kg could effectively suppress the tumor growth, promote the maturation of dendritic cells, and trigger the adaptive T cell response. Combined with programmed death-ligand 1 (PD-L1) immune checkpoint blockade immunotherapy, the iRGD-bcc-USINP-mediated ferroptosis therapy greatly potentiated the immune response and developed strong immune memory. In addition, these USINPs were quickly renal excreted with no side effects in normal tissues. These iRGD-bcc-USINPs provide a simple, safe, effective, and selectively tumor-responsive Fe(0) delivery system for ferroptosis-based immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


