hif -1α诱导的星形细胞d -多巴胺自变性酶激活脊髓损伤后的小胶质细胞炎症反应。
IF 3.6
2区 医学
Q1 PATHOLOGY
引用次数: 0
摘要
脊髓损伤通常导致严重缺氧和神经炎症过度激活,从而加重神经病理和神经功能障碍。D-dopachrome tautoerase (D-DT)是巨噬细胞迁移抑制因子(MIF)的同源物,是参与多种组织炎症性疾病的关键促炎介质。然而,其与缺氧的关系以及对脊髓损伤后神经炎症的潜在影响尚不清楚。本研究检测脊髓损伤后病变部位D-DT、p65NF-κB及下游促炎因子TNF-α、IL-1β、IL-6的动态表达。应用D-DT抑制剂4-CPPC评价其对损伤组织炎症反应的影响。通过体外细胞模型,研究了D-DT介导的小胶质细胞活化及其调控机制,发现D-DT可驱动CD74/MAPKs信号通路激活小胶质细胞炎症。大鼠D-DT启动子分析确定了HIF-1α的结合元件。缺氧或DMOG刺激星形胶质细胞可有效促进HIF-1α和D-DT的表达,而星形胶质细胞条件培养基(ACM)培养小胶质细胞可增加TNF-α、IL-1β和IL-6的产生。脊髓损伤后用4-CPPC或LW6药物治疗可显著促进大鼠运动功能的恢复。这些结果揭示了D-DT的一种新的神经病理功能,这可能有助于开发靶向神经炎症的潜在药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
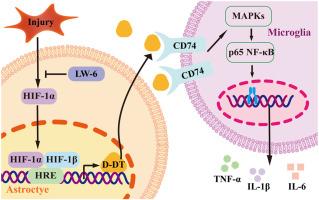
Hypoxia-Inducible Factor-1α–Induced Astrocytic D-Dopachrome Tautomerase Activates Microglial Inflammatory Response following Spinal Cord Injury
Spinal cord injury (SCI) often results in severe hypoxia and excessive activation of neuroinflammation, which aggravates neuropathology and neurologic dysfunction. D-dopachrome tautomerase (D-DT), the homolog of macrophage migration inhibitory factor, is a key proinflammatory mediator implicated in inflammatory diseases of multiple tissues. However, its relation with hypoxia and the potential impact on neuroinflammation following SCI remain elusive. Herein, the dynamic expression of D-DT, p65NF-κB, and the downstream proinflammatory cytokines tumor necrosis factor-α, IL-1β, and IL-6 at the lesion site was determined following SCI. D-DT inhibitor 4-(3-carboxyphenyl)-2,5pyridinedicarboxylic acid was applied to evaluate its effects on the inflammatory responses of the injured tissues. By using an in vitro cell model, the D-DT–mediated activation of microglia and the underlying regulatory mechanism were also investigated, showing that CD74/mitogen-activated protein kinase signaling was driven by D-DT to activate microglial inflammation. Analysis of rat D-DT promoter identified the binding element of hypoxia-inducible factor-1α. Hypoxia or dimethyloxallyl glycine stimulation of astrocytes was shown efficient in promoting the expression of hypoxia-inducible factor-1α and D-DT, whereas incubation of the microglia with the astrocytes’ conditional medium increased the production of tumor necrosis factor-α, IL-1β, and IL-6. Pharmacologic treatment of the subjects with 4-(3-carboxyphenyl)-2,5pyridinedicarboxylic acid or LW6 following SCI remarkably promoted the recovery of rat locomotor function. The results have presented a novel neuropathologic function of D-DT, which might be beneficial for development of potential drug targeting neuroinflammation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


