烯烃自由基1,4-酰基氰化反应合成ζ-酮腈
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-09-05
DOI:10.1039/d5qo00744e
引用次数: 0
摘要
双组分烯烃偶联反应是合成复杂分子结构的有效平台。本文利用碳自由基前体之间活化能垒的差异和自由基之间的极性匹配,首次利用两个相同的烯烃进行1,4-酰基氰基烷基化,合成了难以接近的ζ-酮腈。在无金属体系中,2-(叔丁基过氧基)-2-甲基丙烷(DTBP)分别激活醛和烷基腈的α-C−H键,生成酰基和氰烷基自由基。反应顺序包括酰基自由基选择性加成到两个相同的烯烃上,然后与氰烷基自由基进行自由基-自由基偶联,从而在简单条件下构建3个C−C键。值得注意的是,当叔烷基醛被使用时,脱羰优先发生,形成烷基自由基,使烯烃的1,4-烷基氰烷基化。机理研究和密度泛函理论(DFT)计算表明,这种1,4-酰基氰化过程的成功是由酰基自由基对烯烃的优先加成和与双组分烯烃加成级联相关的热力学稳定性决定的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
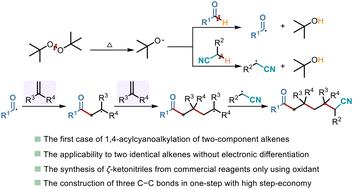
Radical 1,4-acylcyanoalkylation of alkenes for the synthesis of ζ-ketonitriles†
Two-component alkene coupling reactions serve as an efficient platform for the synthesis of complex molecular architectures. Leveraging the differences in activation energy barriers among carbon radical precursors and the polarity matching between radicals, this work reports the first example of radical 1,4-acylcyanoalkylation to synthesize challenging-to-access ζ-ketonitriles using two identical alkenes. In the metal-free system, 2-(tert-butylperoxy)-2-methylpropane (DTBP) respectively activates α-C–H bonds of aldehydes and alkyl nitriles to generate acyl and cyanoalkyl radicals. The reaction sequence involves selective radical addition of the acyl radical to two identical alkenes, followed by radical–radical coupling with the cyanoalkyl radical, thereby constructing three C–C bonds under simple conditions. Remarkably, when tertiary alkyl aldehydes are employed, decarbonylation preferentially occurs to form alkyl radicals, enabling 1,4-alkylcyanoalkylation of alkenes. Mechanistic studies and density functional theory (DFT) calculations reveal that the success of this 1,4-acylcyanoalkylation process is governed by both the preferential addition of acyl radicals to alkenes and the thermodynamic stability associated with the two-component alkene addition cascade.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


