阻断癌细胞表面TIP1的功能域可通过β-catenin/Wnt信号通路上调Midkine。
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
癌细胞表现出的耐药性仍然是治疗方法无法提高癌症患者生存率的主要原因之一。当前非小细胞肺癌(NSCLC)治疗方法治疗效果的边际改善要求新的治疗策略。税收相互作用蛋白-1 (TIP1)是一个辐射诱导的分子靶标,参与多种癌症途径。TIP1的表达与NSCLC患者的低生存率相关。阻断TIP1功能域的抗体可降低细胞增殖并使癌细胞对辐射敏感。在用抗tip1抗体处理的细胞的蛋白质组学分析中,观察到Midkine (MDK)增加了10倍。在TIP1阻断后,Wnt信号激活导致MDK mRNA和蛋白水平上调。在抗tip1抗体治疗后,基因沉默的β-catenin取消了MDK的诱导。抑制TIP1和MDK可以降低细胞的集落形成能力,这表明MDK的上调可能是癌细胞对抗抗TIP1抗体的抗增殖能力的一种策略。共同靶向细胞表面TIP1和MDK可能是治疗非小细胞肺癌的有效策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
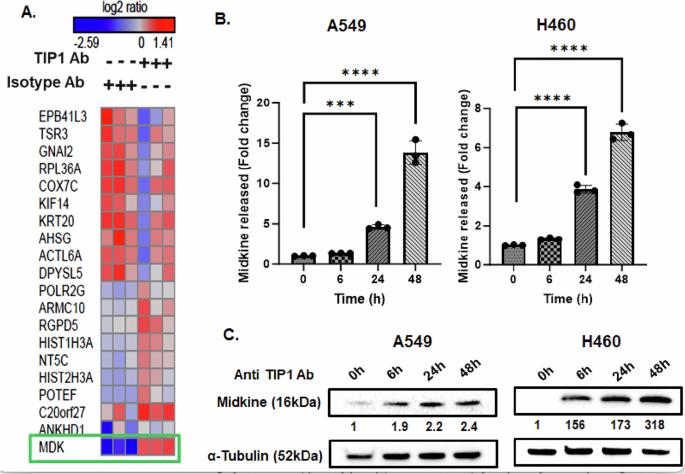
Blocking the functional domain of cancer cell surface TIP1 upregulates Midkine via the β-catenin/Wnt signaling pathway
Drug resistance exhibited by cancer cells remains one of the primary reasons for the failure of therapeutic approaches to increase the survival of cancer patients. Marginal improvement in therapeutic efficacy with current treatment approaches for non-small cell lung cancer (NSCLC) mandates new treatment strategies. Tax interacting Protein-1 (TIP1) is a radiation-inducible molecular target involved in various cancer pathways. TIP1 expression correlates with poor survival in NSCLC patients. Antibody blocking the functional domain of TIP1 reduced cell proliferation and sensitized cancer cells to radiation. A ten-fold increase in Midkine (MDK) was observed in the proteomic analysis of cells treated with anti-TIP1 antibody. Wnt signaling activation led to MDK upregulation at the mRNA and protein levels following TIP1 blockade. Genetic silencing of β-catenin abrogated the induction of MDK following anti-TIP1 antibody treatment. Inhibiting TIP1 along with MDK showed a reduction in the colony-forming capability of the cells, indicating that MDK upregulation might be a strategy employed by cancer cells to combat the anti-proliferative capabilities of the anti-TIP1 antibody. Co-targeting cell surface TIP1 and MDK may be an effective therapeutic strategy for NSCLC patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


