铑催化的单碳插入到1,3-二烯
IF 7.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
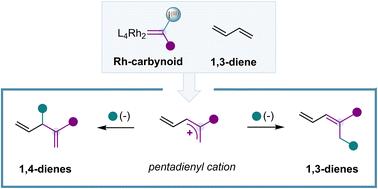
在这里,我们报道了第一个用Rh(II)-羰基化合物催化插入1,3-二烯的单碳。骨架编辑过程是基于催化生成的rh -羰基,促进阳离子单价碳单元(+C - r)插入到1,3-二烯的C(sp2) -C (sp2)键中,导致戊二烯基阳离子。对后者的区域选择性攻击导致形成多取代的1,3-二烯或1,4-二烯与广泛的碳和杂原子亲核试剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Rh-catalysed single-carbon insertion to 1,3-dienes†
Herein, we report the first catalytic single-carbon insertion to 1,3-dienes with Rh(II)-carbynoids. The skeletal editing process is based on the catalytic generation of a Rh-carbynoid that promotes the insertion of a cationic monovalent carbon unit (:+C–R) into the C(sp2)–C(sp2) bond of the 1,3-diene, leading to a pentadienyl cation. Regioselective attack on the latter species leads to the formation of multi-substituted 1,3-dienes or 1,4-dienes with a broad range of carbon and heteroatomic nucleophiles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


