抑制rev - erba表达可防止机械负荷引起的软骨时钟破坏和骨关节炎进展
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
摘要
昼夜节律钟维持包括关节软骨在内的外周组织的内稳态。软骨作为一个高度机械负荷的组织,经历着每天有节奏的机械负荷活动/休息周期模式,这给软骨细胞提供了外部时间线索。考虑到负载模式驱动的软骨时钟,我们假设异常的机械负载是骨关节炎(OA)的一个主要危险因素,可以破坏软骨时钟,进一步促进OA的进展。方法采用无创体内机械加载系统和PER2Luc报告小鼠进行离体生物发光记录。在1.0 MPa静态压缩小鼠原代软骨细胞中进行RNA测序,鉴定出核心时钟分子rev - erba,这在人和小鼠OA软骨样品中得到证实。用Rev-erbα小干扰RNA (si-Rev-erbα)处理小鼠软骨细胞,并在小鼠关节内注射携带Rev-erbα特异性短发夹RNA (AAV-shRev-erbα)的腺相关病毒以敲低Rev-erbα。通过RNA测序数据分析rev - erba调控的相关信号通路。在小鼠机械超载后,腹腔注射特异性rev - erba拮抗剂SR8278进行OA治疗。结果过度的机械负荷破坏了关节软骨的昼夜节律。OA软骨中核心时钟分子REV-ERBα升高,抑制REV-ERBα可减轻压迫性软骨细胞功能障碍。U0126或SB203580抑制MAPK-MYC通路可减弱压缩诱导的REV-ERBα上调和软骨时钟破坏。最后,SR8278对REV-ERBα表达的药理学抑制恢复了异常负荷时的软骨时钟,并减缓了OA的进展。结论rev - erba是机械负荷引起的昼夜节律紊乱与OA病理相关的关键因素。这项研究阐明了超载下昼夜节律受损的基本机制,并为OA的治疗提供了可能有效的治疗方法。以时钟为基础的治疗药物SR8278或MAPK-MYC途径抑制剂抑制REV-ERBα表达可以改善机械性超载引起的软骨和OA变性的昼夜节律中断,表明OA治疗的临床转化潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
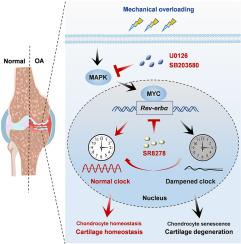
Inhibiting the REV-ERBα expression protects against mechanical overloading-induced cartilage clock disruption and osteoarthritis progression
Background
The circadian clock maintains homeostasis in peripheral tissues, including articular cartilage. Cartilage as a highly mechanical loaded tissue experiences diurnal rhythmic mechanical loading activity/rest cycle patterns, which gives external time cue on chondrocytes. Given the cartilage clock driven by loading patterns, we hypothesize that abnormal mechanical loading, a major risk factor for osteoarthritis (OA), can disrupt the cartilage clock, further contributing to OA progression.
Methods
We used both noninvasive in vivo mechanical loading system and PER2Luc reporter mice for ex vivo bioluminescence recording. RNA sequencing was performed in mouse primary chondrocytes treated with 1.0 MPa static compression, and identified core clock molecule REV-ERBα, which was confirmed in human and murine OA cartilage samples. Chondrocytes were treated with Rev-erbα small interfering RNA (si-Rev-erbα), and adeno-associated virus carrying Rev-erbα-specific short hairpin RNA (AAV-shRev-erbα) was injected intra-articularly in mice to knock down Rev-erbα. Relevant signaling pathways regulating REV-ERBα were analyzed by RNA sequencing data. Intraperitoneal injection of SR8278, a specific REV-ERBα antagonist, was performed in mice after mechanical overloading for OA treatment.
Results
Excessive mechanical loading disrupted the circadian rhythm of articular cartilage. The core clock molecule REV-ERBα was increased in OA cartilage and knockdown of Rev-erbαalleviated compression-induced chondrocyte dysfunction. Inhibition of MAPK-MYC pathway by U0126 or SB203580 attenuated compression-induced REV-ERBα up-regulation and cartilage clock disruption. Finally, pharmacological inhibition of REV-ERBα expression by SR8278 restored cartilage clock upon abnormal loading and mitigated OA progression.
Conclusions
REV-ERBα is a key factor in the association between mechanical overloading-induced circadian disruption and OA pathology. This study illustrates the essential mechanism of impaired circadian rhythm under overloading and provides a possibly impactful therapeutic approach for the treatment of OA.
The Translational Potential of this Article
Inhibition REV-ERBα expression by clock-based therapeutic drug SR8278 or MAPK-MYC pathway inhibitors could ameliorate mechanical overloading-induced circadian disruption of cartilage and OA degeneration, indicating a clinical conversion potential for OA treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


