钴催化内炔与环丁烯的区域、非映对和对映选择性还原偶联。
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文报道了钴催化的内炔和未活化环丁烯的不对称烯-炔还原偶联反应。该反应产生密集功能化的手性乙烯基环丁烷,产率高达92%,具有良好的绝对和相对立体控制性(>99% ee, >20:1 dr, >20:1 E/Z), >20:1区域选择性高。放大后的反应和合成后的衍生化反应进一步证明了所设计方案的有效性。初步的机制研究表明,锌介导的低价钴(I)配合物的生成、氧化炔炔环化和质子化是关键的机制步骤。本文章由计算机程序翻译,如有差异,请以英文原文为准。

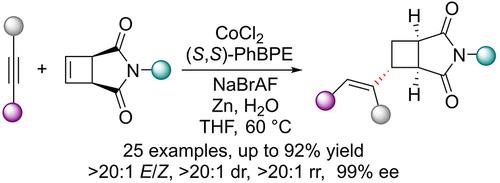
Cobalt-Catalyzed Regio-, Diastereo-, and Enantioselective Reductive Coupling of Internal Alkynes with Cyclobutenes
Herein, we report the cobalt-catalyzed asymmetric ene–yne reductive coupling of internal alkynes and unactivated cyclobutenes. The reaction produced densely functionalized chiral vinyl cyclobutanes in up to 92% yields with excellent absolute and relative stereocontrol (>99% ee, >20:1 dr, and >20:1 E/Z), and high >20:1 regioselectivity. The scaled-up reaction and the postsynthetic derivatizations further elucidated the efficiency of the designed protocol. The preliminary mechanistic investigations suggested the involvement of zinc-mediated low-valent cobalt(I) complex generation, oxidative ene–yne cyclization, and protonation as the key mechanistic steps.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


