基于3WJ的适配体靶向抗mir RNA构建作为治疗慢性肾脏疾病的新方法
IF 4.5
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
慢性疾病(CKD)的治疗是医疗保健领域的一个巨大挑战,需要一种创新的方法来解决其复杂性。RNA纳米技术在过去几年中迅速兴起并受到关注,因为它具有显著的治疗能力。因此,本研究旨在设计、构建和表征一种基于噬菌体phi29包装RNA三向结(pRNA-3WJ)的多功能(anti-miR-34a DNA适配体-肾脏靶向)RNA纳米颗粒(RNPs),并探讨其在小鼠CKD模型中的体内毒性和治疗潜力。在用健康小鼠证实制备的核心3WJ (3WJ)和治疗性3WJ (3WJ- kapt /anti-miR-34a) RNPs对肾组织的安全性和特异性靶向能力后,用腺嘌呤诱导C57BL/6小鼠CKD。通过单次静脉注射3WJ或3WJ- kapt /anti-miR-34a治疗CKD小鼠。治疗后4周,每组每周随机取5只小鼠取样。抗miR-34a 3WJ-RNPs在利用DNA适体靶向肾脏方面显示出稳定性、安全性和有效性,通过靶向肾组织中的miR-34a, 3WJ-Kapt/anti-miR-34a抑制促纤维化基因的表达并诱导抗纤维化通路的表达。我们目前的研究为通过3WJ-RNPs靶向肾组织中的miR-34a治疗肾纤维化和CKD提供了初步和开创性的证据。CKD小鼠的肾纤维化通路,包括TGF-β、FGF2和WNT/β-catenin通路,表现出明显的时间依赖性上调。相同的小鼠显示抗纤维化途径的肾脏表达受到抑制,包括α和β Klotho, SMAD7和SIRT1。制备的anti-miR-34a 3WJ-RNPs在使用DNA适配体靶向肾脏方面显示出稳定性、安全性和有效性。3WJ-Kapt/anti-miR-34a通过靶向肾组织中的miR-34a,抑制促纤维化基因的表达,诱导抗纤维化通路的表达。我们目前的研究为通过3WJ-RNPs靶向肾组织中的miR-34a治疗肾纤维化和CKD提供了初步和开创性的证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
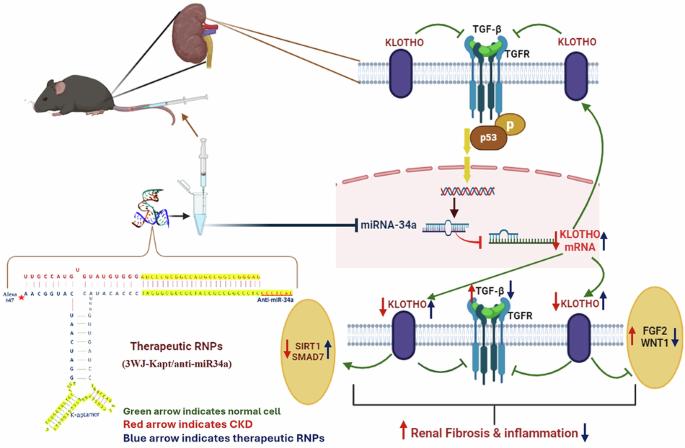
Aptamer-targeted anti-miR RNA construct based on 3WJ as a new approach for the treatment of chronic kidney disease in an experimental model
The treatment of chronic disease (CKD) is a great challenge in healthcare that requires an innovative approach to address its complex nature. RNA nanotechnology has emerged rapidly and received attention in the last few years because of its significant aptitude for therapies. Hence, the present study aimed to design, construct, and characterize a multifunctional (anti-miR-34a DNA aptamer-kidney targeted) RNA nanoparticle (RNPs) based on bacteriophage phi29 packaging RNA three-way junction (pRNA-3WJ), and then explore their in vivo toxicity and therapeutic potentials in mice model of CKD. After confirming the safety and specific targeting capability of the prepared core 3WJ (3WJ) and the therapeutic 3WJ (3WJ-Kapt/anti-miR-34a) RNPs to renal tissue using healthy mice, CKD was induced in C57BL/6 mice using adenine. CKD mice were treated with a single intravenous injection of 3WJ or 3WJ-Kapt/anti-miR-34a. Every week, 5 mice of each group were selected randomly for sample collection for 4 weeks post-treatment. The anti-miR-34a 3WJ-RNPs have shown stability, safety, and efficacy in renal targeting using DNA aptamer, by targeting miR-34a in renal tissue, 3WJ-Kapt/anti-miR-34a suppressed profibrotic gene expression and induced anti-fibrotic pathways’ expression. Our present study provides preliminary and pioneering evidence for the promising treatment of renal fibrosis and CKD through targeting miR-34a in the renal tissue by 3WJ-RNPs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


