热细胞活动弧菌核糖5-磷酸异构酶B的突变和结构分析:转化效率与底物结合袋构象的关系
IF 3.7
3区 生物学
Q2 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
d -醛糖是一种罕见的己糖,在食品、医药等领域具有多种潜在的应用前景。核糖5-磷酸异构酶(RPI)在D-allose的合成中起关键作用。来自热细胞活动弧菌的AtRpiB可以将D-psicose转化为D-allose;然而,它的稳定性,最佳温度和转换效率的改进是必要的。本研究旨在以D-psicose为底物考察AtRpiB的催化性能、稳定性和底物结合亲和力。采用不同的策略,选取10个氨基酸残基构建了82个单突变体文库。9个单突变体表现出较高的D-allose转化率。通过9个成功的单突变位点组合,构建了27个双突变体。值得注意的是,突变体R109W/R132Q、S39I/R109F和S39V/R109F在40℃、60℃和80℃下的酶活性分别提高了1.39倍、1.58倍和1.9倍。在40°C下,S39V/R109F突变体在18 小时后将100 g/L D-psicose转化为38.21 g/L D-allose,这是迄今为止报道的RpiB在D-allose生产中的最高转化率。酶学特性、结构分析和分子动力学模拟表明,AtRpiB的氨基酸组成和3、9、10环构象调整的变化显著影响底物和产物进入活性口袋的进出、转化效率和酶的稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
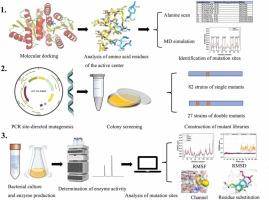
Mutational and structural analysis of ribose 5-phosphate isomerase B from Acetivibrio thermocellus: relationship between transformation efficiency and substrate binding pocket conformation
D-allose is a rare hexose sugar with a variety of potential application in food, medicine and other fields. Ribose 5-phosphate isomerase(RPI) plays a pivotal role in the synthesis of D-allose. AtRpiB from Acetivibrio thermocellus can convert D-psicose to D-allose; however, improvements in its stability, optimal temperature, and conversion efficiency are necessary. This study aimed to investigate the catalytic properties, stability, and substrate-binding affinity of AtRpiB using D-psicose as the substrate. Using various strategies, 10 amino acid residues were selected to construct a library of 82 single mutants. Nine single mutants showed high conversion rates of D-allose. Furthermore, 27 double mutants were constructed by combining nine successful single mutation sites. Notably, the mutants R109W/R132Q, S39I/R109F, and S39V/R109F showed a 1.39-fold increase in enzyme activity at 40 ºC, 1.58-fold increase at 60 ºC, and 1.9-fold increase at 80 ºC, respectively. The S39V/R109F mutant converted 100 g/L D-psicose to 38.21 g/L D-allose after 18 hours at 40°C, the highest reported conversion rate for RpiB in D-allose production to date. Analysis of enzymatic characteristics, structure, and molecular dynamics simulations revealed that changes in the amino acid composition and conformational adjustments in loops 3, 9, and 10 of AtRpiB significantly affected the entry and exit of substrates and products into the active pocket, conversion efficiency, and enzyme stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Enzyme and Microbial Technology
生物-生物工程与应用微生物
CiteScore
7.60
自引率
5.90%
发文量
142
审稿时长
38 days
期刊介绍:
Enzyme and Microbial Technology is an international, peer-reviewed journal publishing original research and reviews, of biotechnological significance and novelty, on basic and applied aspects of the science and technology of processes involving the use of enzymes, micro-organisms, animal cells and plant cells.
We especially encourage submissions on:
Biocatalysis and the use of Directed Evolution in Synthetic Biology and Biotechnology
Biotechnological Production of New Bioactive Molecules, Biomaterials, Biopharmaceuticals, and Biofuels
New Imaging Techniques and Biosensors, especially as applicable to Healthcare and Systems Biology
New Biotechnological Approaches in Genomics, Proteomics and Metabolomics
Metabolic Engineering, Biomolecular Engineering and Nanobiotechnology
Manuscripts which report isolation, purification, immobilization or utilization of organisms or enzymes which are already well-described in the literature are not suitable for publication in EMT, unless their primary purpose is to report significant new findings or approaches which are of broad biotechnological importance. Similarly, manuscripts which report optimization studies on well-established processes are inappropriate. EMT does not accept papers dealing with mathematical modeling unless they report significant, new experimental data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


