HIV潜伏期的数学建模和机制用于个性化抗潜伏期治疗。
IF 3.5
2区 生物学
Q1 MATHEMATICAL & COMPUTATIONAL BIOLOGY
引用次数: 0
摘要
联合抗逆转录病毒治疗控制人类免疫缺陷病毒-1 (HIV),但不能根除免疫细胞中的潜伏前病毒,这些病毒在治疗中断后会重新激活。像“休克-杀伤”这样的抗潜伏期疗法正在开发中,但由于潜伏期机制的复杂性,尚未取得成功。这篇综述讨论了通过数学模型理解HIV潜伏期的最新进展,涵盖了预测潜伏期逆转的关键调控因子和模型,强调了指导未来治疗方法的差距。本文章由计算机程序翻译,如有差异,请以英文原文为准。
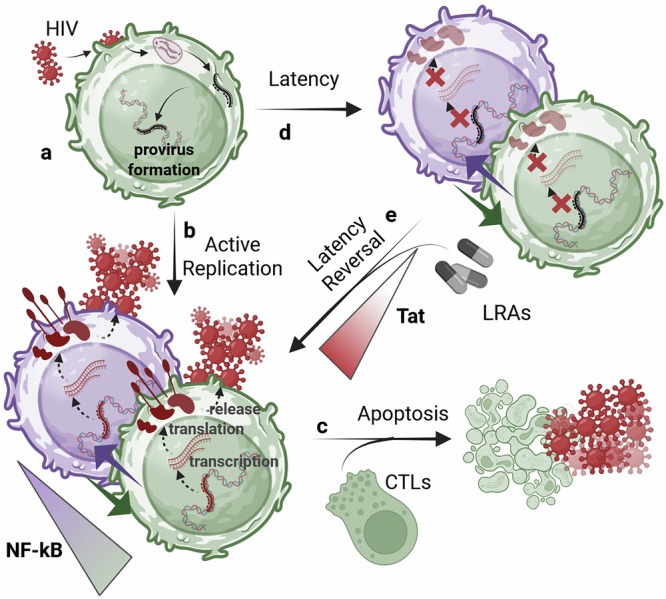
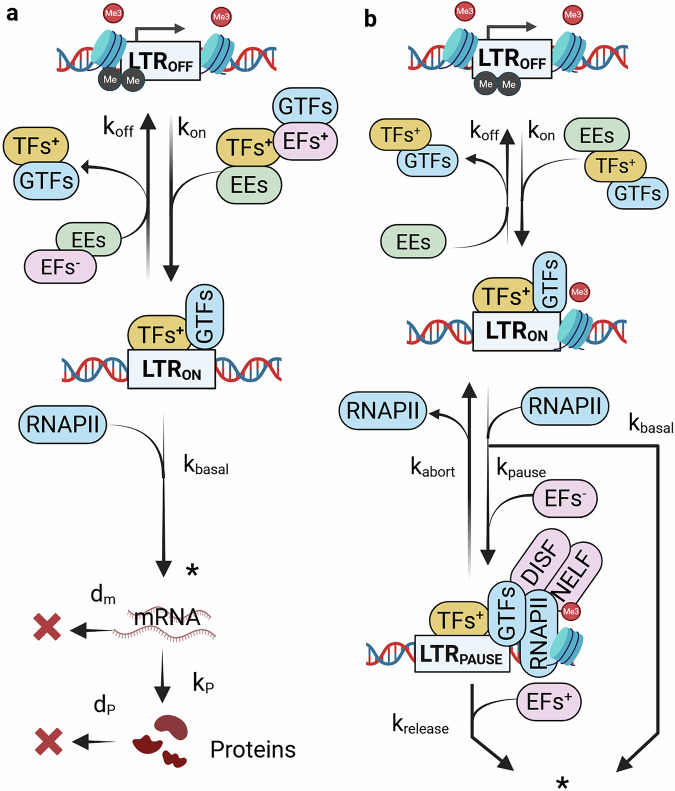
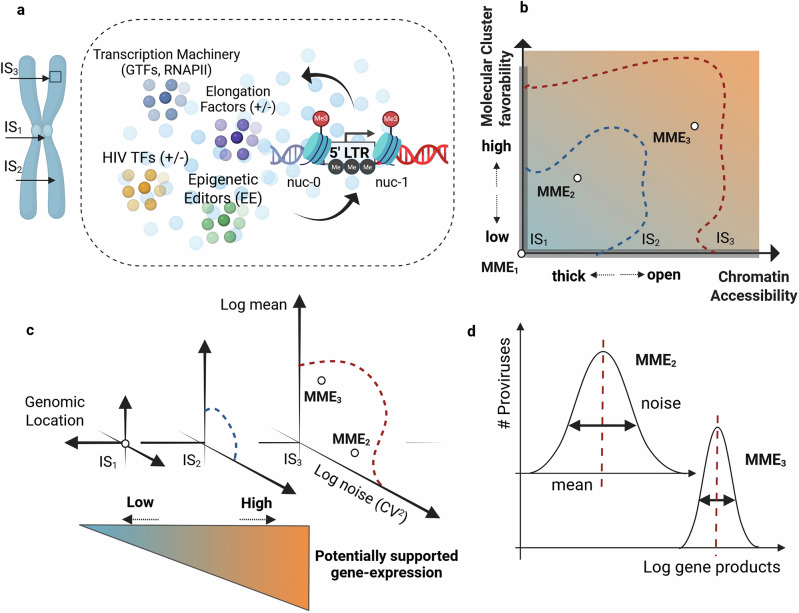
Mathematical modeling and mechanisms of HIV latency for personalized anti latency therapies.
Combination antiretroviral therapy controls human immunodeficiency virus-1 (HIV) but cannot eradicate latent proviruses in immune cells, which reactivate upon treatment interruption. Anti-latency therapies like "shock-and-kill" are being developed but are yet to succeed due to the complexity of latency mechanisms. This review discusses recent advances in understanding HIV latency via mathematical modeling, covering key regulatory factors and models to predict latency reversal, highlighting gaps to guide future therapeutic approaches.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

NPJ Systems Biology and Applications
Mathematics-Applied Mathematics
CiteScore
5.80
自引率
0.00%
发文量
46
审稿时长
8 weeks
期刊介绍:
npj Systems Biology and Applications is an online Open Access journal dedicated to publishing the premier research that takes a systems-oriented approach. The journal aims to provide a forum for the presentation of articles that help define this nascent field, as well as those that apply the advances to wider fields. We encourage studies that integrate, or aid the integration of, data, analyses and insight from molecules to organisms and broader systems. Important areas of interest include not only fundamental biological systems and drug discovery, but also applications to health, medical practice and implementation, big data, biotechnology, food science, human behaviour, broader biological systems and industrial applications of systems biology.
We encourage all approaches, including network biology, application of control theory to biological systems, computational modelling and analysis, comprehensive and/or high-content measurements, theoretical, analytical and computational studies of system-level properties of biological systems and computational/software/data platforms enabling such studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


