橄榄苦苷调节泛素化介导的Mcl-1周转并显示抗肿瘤活性。
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
口腔鳞状细胞癌(OSCC)是最常见的口腔恶性肿瘤类型,在全球范围内具有很高的患病率。尽管OSCC治疗不断进步,但其5年生存率仍保持在50%左右,这表明迫切需要开发新的有效治疗策略。在这里,我们专注于骨髓性白血病1 (Mcl-1),一个众所周知的人类癌症的致癌驱动因素,并通过体外和体内分析系统地探索橄榄苷(Ole)的治疗潜力。我们的研究结果表明,Ole对OSCC细胞活力的抑制呈剂量依赖性。在机制上,Ole通过抑制Akt-GSK3β-Mcl-1通路,增强β-TRCP与Mcl-1的协同作用,促进β-TRCP介导的Mcl-1泛素化,最终导致Mcl-1降解。此外,敲低β-TRCP可减轻Ole对OSCC细胞的抑制作用。与我们基于细胞的实验一致,动物研究表明Ole治疗显著延迟肿瘤生长,而不会对重要器官造成毒性。此外,无论是单独使用还是与放疗联合使用,Ole都有效地克服了OSCC细胞的放射耐药。我们的研究结果表明,Ole是一种很有前景的抗肿瘤药物,能够靶向Mcl-1治疗OSCC。本文章由计算机程序翻译,如有差异,请以英文原文为准。
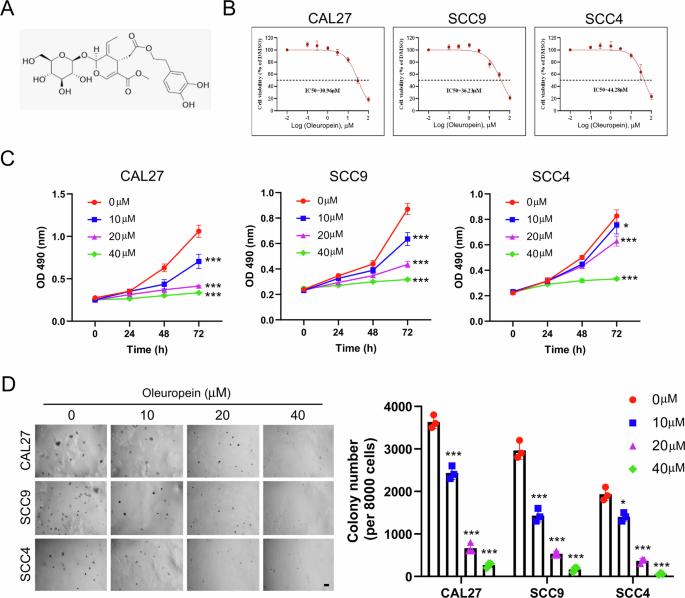
Oleuropein regulates ubiquitination-mediated Mcl-1 turnover and exhibits antitumor activity
Oral squamous cell carcinoma (OSCC) represents the most common type of malignant oral tumor, with a high prevalence globally. Despite continual advancements in OSCC treatment, the 5-year survival rate remains around 50%, highlighting an urgent need for the development of new and effective therapeutic strategies. Here, we focused on myeloid leukemia 1 (Mcl-1), a well-known oncogenic driver in various human cancers, and systematically explored the therapeutic potential of oleuropein (Ole) through in vitro and in vivo analyses. Our findings demonstrated that Ole suppressed OSCC cell viability dose-dependently. Mechanistically, Ole facilitated β-TRCP-mediated ubiquitination of Mcl-1 by inhibiting the Akt-GSK3β-Mcl-1 pathway and enhancing the collaboration between β-TRCP and Mcl-1, ultimately leading to Mcl-1 degradation. Furthermore, the knockdown of β-TRCP mitigated the inhibitory effects of Ole on OSCC cells. In agreement with our cell-based experiments, animal studies showed that Ole treatment significantly delayed tumor growth without causing toxicity to vital organs. Additionally, whether used alone or combined with radiation, Ole effectively overcame radioresistance in OSCC cells. Our results suggest that Ole is a promising anti-tumor agent capable of treating OSCC by targeting Mcl-1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


