局部递送靶向IL-33的人单域抗体抑制粘膜炎症。
IF 19.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
由于生物疗法穿透上皮屏障的能力有限,解决眼表或呼吸系统粘膜炎症性疾病仍然是一个艰巨的挑战。在这项研究中,我们探索了人类单域抗体(UdAbs)作为靶向调节白细胞介素-33 (IL-33)治疗两种粘膜相关炎症疾病的局部治疗方法的潜力。抗IL-33 UdAb A12显示出IL-33介导的信号通路的有效抑制,尽管没有有效阻断IL-33受体的相互作用。与抗il -33对照IgG itepekimab相比,局部递送A12导致体内角膜浓度显著升高,但可忽略眼部穿透。此外,A12通过发挥抗炎作用显著改善干眼病的严重程度。此外,在另一种过敏性哮喘小鼠模型中,吸入A12可显著降低整体肺部炎症。我们的研究结果揭示了UdAbs在非侵入性局部递送后穿透粘膜屏障的能力,突出了它们作为调节粘膜炎症的创新治疗策略的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
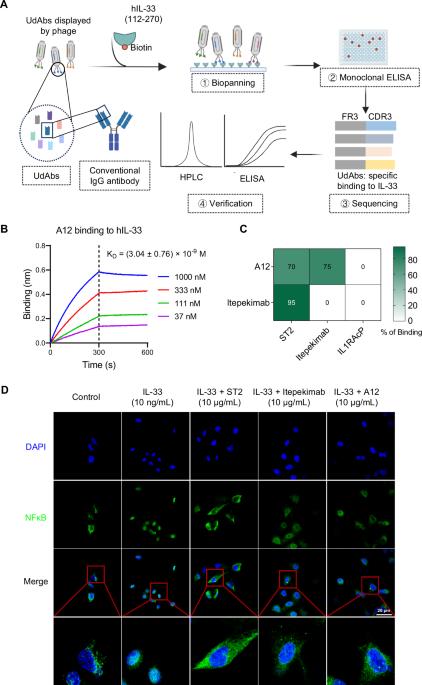
Topical delivery of a human single-domain antibody targeting IL-33 to inhibit mucosal inflammation
Addressing mucosal inflammatory disorders in the ocular surface or respiratory system remains a formidable challenge owing to the limited penetration of biological therapeutics across epithelial barriers. In this study, we explored the potential of human single-domain antibodies (UdAbs) as topical therapeutics for the targeted modulation of interleukin-33 (IL-33) in two mucosal-associated inflammatory disorders. The anti-IL-33 UdAb A12 demonstrated potent inhibition of the IL-33-mediated signaling pathway, despite not potently blocking the IL-33 receptor interaction. Compared with the anti-IL-33 control IgG itepekimab, the topical delivery of A12 resulted in significantly elevated corneal concentrations in vivo, which resulted in negligible ocular penetration. Moreover, A12 considerably ameliorated dry eye disease severity by exerting anti-inflammatory effects. Furthermore, in another murine model of allergic asthma, inhaled A12 substantially reduced overall lung inflammation. Our findings revealed the capacity of UdAbs to penetrate mucosal barriers following noninvasive localized delivery, highlighting their potential as an innovative therapeutic strategy for modulating mucosal inflammation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


