小鼠肾脏中卵泡蛋白缺失通过异常的TFEB激活导致Henle环的膀胱形成。
IF 3.6
2区 医学
Q1 PATHOLOGY
引用次数: 0
摘要
哺乳动物的肾脏包含许多与收集管相连的肾单位,每个肾单位由肾小球、近端小管、亨勒袢(LoH)和远端小管组成。卵泡蛋白(Folliculin, FLCN)是BHD综合征(birt - hogg - dub本文章由计算机程序翻译,如有差异,请以英文原文为准。
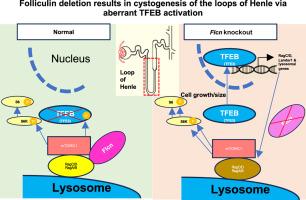
Folliculin Deletion in the Mouse Kidney Results in Cystogenesis of the Loops of Henle via Aberrant TFEB Activation
The mammalian kidney contains numerous nephrons connected to the collecting ducts, and each nephron consists of a glomerulus, a proximal tubule, the loop of Henle (LoH), and a distal tubule. Folliculin (FLCN) is a causative gene for Birt-Hogg-Dubé syndrome, which is characterized by a variety of manifestations, including renal cysts and cancer. Although deletion of Flcn in the mouse collecting duct and distal nephron leads to cyst formation, its precise role in the entire nephron remains unclear. Herein, nephron-specific Flcn knockout mice exhibited cystogenesis along the entire nephron segments, most prominent in the LoH, preceded by an irregularly shaped lumen lined by enlarged epithelia. Single-cell RNA sequencing revealed many up-regulated genes, especially in the knockout LoH. These genes included those related to lysosomal activity and mammalian target of rapamycin complex 1 activation and are likely targets of transcription factor E3 (TFE3)/transcription factor EB (TFEB). Although the double Flcn/Tfe3 knockout only ameliorated the glomerular cysts, the double Flcn/Tfeb knockout largely reversed most of the phenotypes along the entire nephron. Thus, Flcn deletion led to cystogenesis via aberrant TFEB activation. These findings show the essential role of the FLCN-TFEB signaling pathway in nephron development, particularly in LoH, and shed light on the pathogenesis of Birt-Hogg-Dubé syndrome.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


