锰配合物催化(脱氢)环化选择性合成2-取代和2,3-二取代4-喹诺酮
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们描述了双功能的NNO-Mn(I)-催化3-苄基-2-苯基喹啉-4(1H)- 1和2-苯基喹啉-4(1H)- 1及其衍生物,通过(脱)氢化环化与不同的氨基苯乙酮醇。为了扩大催化方案的通用性,我们进行了具有生物活性化合物的合成后修饰,并合成了具有抗生素特性的4-喹诺酮类药物。通过控制实验和动力学实验验证了机理分析,结果表明,c -烷基化比n -烷基化更有利于这两种杂环化合物的合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
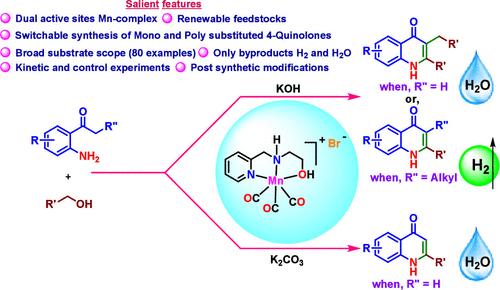
Manganese Complex-Catalyzed (De)hydrogenative Cyclization toward the Selective Synthesis of 2-Substituted and 2,3-Disubstituted 4-Quinolones
Herein, we describe bifunctional NNO-Mn(I)-catalyzed switchable synthesis of 3-benzyl-2-phenylquinolin-4(1H)-one and 2-phenylquinolin-4(1H)-one and their derivatives via (de)hydrogenative annulation of a host of alcohols with diverse amino acetophenones. To expand the versatility of our catalytic protocol, we conducted postsynthetic modification featuring bioactive compounds and synthesized 4-quinolones with antibiotic properties. Control and kinetic experiments were carried out to validate the mechanistic analysis, demonstrating that C-alkylation is preferred over N-alkylation in the synthesis of both heterocycles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


