直接pd催化脂肪族羧酸β-C(sp3) -H羟基化
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
由于β-羟基羰基支架存在于各种天然产物和生物活性分子中,因此具有至关重要的意义。在这项研究中,我们提出了直接钯催化脂肪族羧酸的β-C(sp3) -H羟基化。所报道的方法采用方便和容易获得的三必和二酚作为氧化剂,并提供了一个有效的,一步的路线,以多种β-羟基酸。关键方面包括该方法与具有挑战性的α-非季羧酸作为底物的相容性以及优异的官能团耐受性。这种新开发的方法有望证明对生成与多学科相关的复合库是有用的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
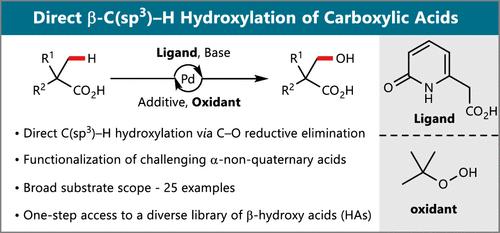
Direct Pd-Catalyzed β-C(sp3)–H Hydroxylation of Aliphatic Carboxylic Acids
β-Hydroxy carbonyl scaffolds are of paramount importance due to their presence in a variety of natural products and bioactive molecules. In this study, we present a direct palladium-catalyzed β-C(sp3)–H hydroxylation of aliphatic carboxylic acids. The reported method employs convenient and readily available TBHP as an oxidant and provides an efficient, one-step route to a diverse range of β-hydroxy acids. Key aspects include the compatibility of this method with challenging α-non-quaternary carboxylic acids as substrates and excellent functional group tolerance. This newly developed method is expected to prove useful for generating compound libraries relevant to multiple disciplines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


