胎盘线粒体DNA突变对婴儿负性情感的影响:母亲终生应激和婴儿性别的调节作用。
摘要
神经精神和行为障碍影响了超过15%的美国儿童,在表现上存在性别差异。产前暴露于社会心理压力可预测不良的神经发育结果,特别是在妊娠期间。机制仍然知之甚少。研究将产前应激暴露与胎盘线粒体DNA (mtDNA)突变负荷联系起来,表明胎盘线粒体功能的破坏可能起作用。我们的概念是胎盘线粒体生物标志物反映了环境诱导的氧化,这可能有助于影响神经发育的机制。此外,由于母亲压力对女性和男性儿童的影响不同,这可能部分解释了儿童早期神经行为结果的性别差异。本研究探讨了胎盘mtDNA突变负荷与婴儿负性情感之间的关系,以及这些关系是否受到母亲终生压力和胎儿性别的影响。在分娩时收集胎盘样本(N = 394),并进行全mtDNA测序以确定基因特异性突变负荷。母亲在孩子6.69±1.61月龄时填写婴儿行为修正问卷(IBQ-R),并得出负面情感因子。我们进行了多变量回归分析来模拟负面情绪与胎盘mtDNA突变负荷的关系,首先调整了儿童性别和母亲年龄、自我报告的种族和教育程度。最后,我们使用交叉产品术语和对比语句检验了母亲压力和胎儿性别对效果的影响。结果表明,MT_CYB区域较高的突变负荷与负性情绪增加呈正相关。值得注意的是,mtDNA区域(MT_DLOOP和MT_ND)、儿童性别和母亲压力之间的相互作用表明,这些区域突变负荷较高的女孩有更大的负性情感增加的风险,特别是在高母亲压力下。这些发现表明,胎盘mtDNA突变负荷可以作为神经发育风险的生物标志物,其性别特异性脆弱性受母体应激的影响。这项研究强调了在理解早期神经发育轨迹时考虑环境因素和性别差异的重要性,以及胎盘作为早期检测和干预工具的潜力。需要进一步的研究来验证这些发现,并探索其对儿童长期发展的影响。胎盘mtDNA细胞色素B区突变负荷的增加与婴儿较高的负情感相关。女孩对线粒体d -环和NADH脱氢酶区域的突变表现出更大的敏感性,与男孩相比,女孩表现出更强的消极情感联系。较高的母亲终生压力放大了线粒体NADH脱氢酶突变负荷对女孩负情感的影响,突出了基因与环境的相互作用。研究结果强调了胎盘在整合影响早期气质的环境和遗传因素方面的作用,以及它作为未来生物标志物的潜在作用。这是第一个将胎盘线粒体DNA突变与不同人群中的婴儿气质联系起来的研究,揭示了性别特异性和压力调节效应。
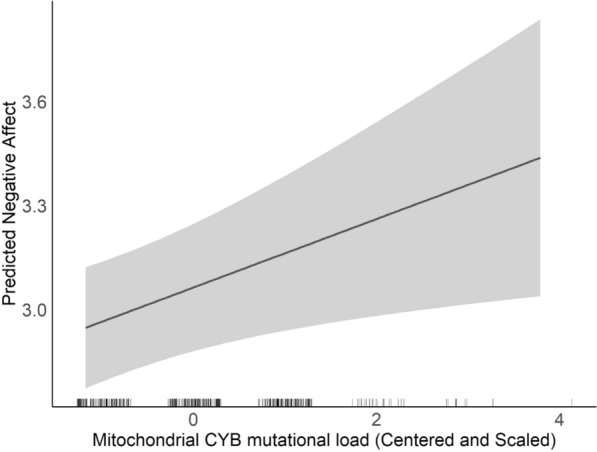
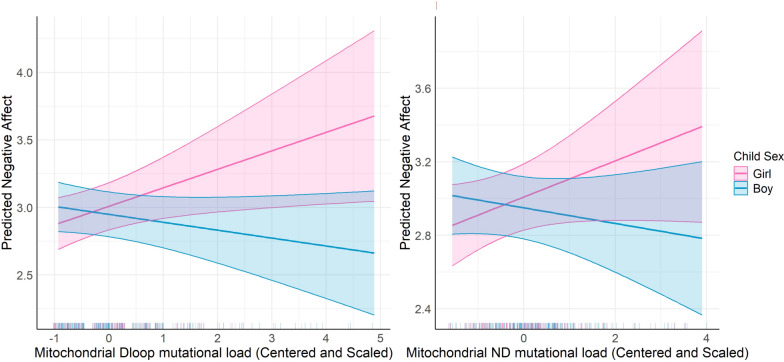
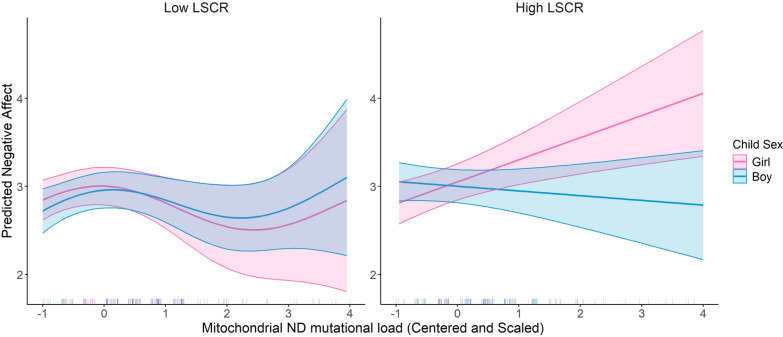
Neuropsychiatric and behavioral disorders impact over 15% of U.S. children, with sex differences in manifestation. Prenatal exposure to psychosocial stress predicts adverse neurodevelopmental outcomes, particularly during gestation. Mechanisms remain poorly understood. Research links prenatal stress exposures with placental mitochondrial DNA (mtDNA) mutational load, suggesting that disrupted mitochondrial placental function may play a role. We conceptualize that placental mitochondrial biomarkers reflect environmentally-induced oxidation that may contribute to mechanisms influencing neurodevelopment. Furthermore, as maternal stress can impact female and male children differently, this may in part explain sex differences in early childhood neurobehavioral outcomes. This study explores the association between placental mtDNA mutational load and negative affectivity in infants, and whether these associations are modified by maternal lifetime stress and fetal sex. Placenta samples (N = 394) were collected at delivery and whole mtDNA sequencing was performed to identify gene-specific mutational loads. Mothers completed the Infant Behavior Questionnaire-Revised (IBQ-R) when children were 6.69 ± 1.61 months of age and the Negative Affectivity factor was derived. Multivariable regression analyses were performed to model Negative Affectivity in relation to placental mtDNA mutational load, first adjusting for child sex and maternal age, self-reported race, and education. Lastly, we examined effect modification by maternal stress and fetal sex using cross-product terms and contrast statements. Results showed that higher mutational load in the MT_CYB region was positively associated with increased negative affectivity. Notably, interactions between mtDNA regions (MT_DLOOP and MT_ND), child sex, and maternal stress revealed that girls with higher mutational loads in these regions were at greater risk for increased negative affectivity, particularly under high maternal stress. These findings suggest that placental mtDNA mutational load could serve as a biomarker for neurodevelopmental risk, with sex-specific vulnerabilities influenced by maternal stress. This study underscores the importance of considering both environmental factors and sex differences in understanding early neurodevelopmental trajectories, and the potential of the placenta as a tool for early detection and intervention. Further research is needed to validate these findings and explore their implications for long-term child development. Highlights Increased mutational load in the cytochrome B region of placental mtDNA is associated with higher infant negative affectivity. Girls exhibited greater sensitivity to mutations in the mitochondrial D-loop and NADH dehydrogenase regions, showing stronger links to negative affectivity compared to boys. Higher maternal lifetime stress amplified the impact of mitochondrial NADH dehydrogenase mutational load on negative affectivity in girls, highlighting gene-environment interactions. Findings underscore the placenta's role in integrating environmental and genetic factors that influence early temperament, and its potential role as a future biomarker. This is the first study connecting placental mitochondrial DNA mutations with infant temperament in a diverse population, revealing sex-specific and stress-modulated effects.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


