级联pd催化的分子间n -芳基化/分子内烯烃碳胺化反应合成双氢吲哚吲哚。
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
钯催化的2-烯丙苯胺与1-溴-2-氯苯衍生物的交叉偶联提供了中高收率的二氢吲哚吲哚,高达15:1博士。转化包括最初的pd催化的n -芳基化,生成取代的二苯胺衍生物,然后通过分子内pd催化的烯碳胺化,通过氮杂环苯并环丁烯中间体生成四环产物。描述了这些反应的范围、机理和立体控制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
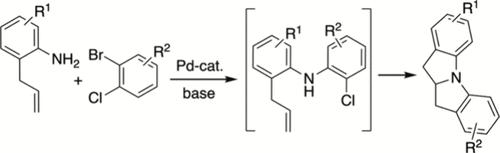
Cascade Pd-Catalyzed Intermolecular N-Arylation/Intramolecular Alkene Carboamination Reactions for the Synthesis of Dihydroindoloindoles.
The palladium-catalyzed cross-coupling of 2-allylanilines with 1-bromo-2-chlorobenzene derivatives provides dihydroindoloindoles in moderate to good yield with up to 15:1 dr. The transformations involve initial Pd-catalyzed N-arylation to generate a substituted diphenylamine derivative, followed by intramolecular Pd-catalyzed carboamination of the alkene via an azapalladabenzocyclobutene intermediate to generate the tetracyclic products. The scope, mechanism, and stereocontrol of these reactions is described.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


