极光激酶B抑制克服met扩增肺癌中met靶向耐药
IF 3.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular cell research
Pub Date : 2025-06-09
DOI:10.1016/j.bbamcr.2025.120001
引用次数: 0
摘要
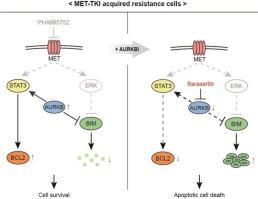
met靶向治疗是met扩增型肺癌患者最有效的治疗方法。然而,获得性耐药是met扩增型肺癌治疗的重大挑战。本研究旨在发现克服met靶向耐药的有效治疗策略。我们首先从MET扩增的肺癌细胞(H1993)中建立了对MET酪氨酸激酶抑制剂(MET- tki) (H1993 PR-S2)耐药的肺癌细胞系。利用抗癌化合物文库进行高通量筛选,发现极光激酶B (AURKB)抑制剂是抑制H1993 PR-S2细胞活力的有效药物。在这些耐药细胞中,p-MET表达显著降低,p-AURKB表达显著升高。此外,在H1993 PR-S2细胞中,stat3激活的基因特征丰富,p-STAT3的表达与AURKB密切相关。在亲本细胞中,AURKB过表达诱导p-STAT3活化,而在H1993 PR-S2细胞中,AURKB敲低可降低p-STAT3的表达。耐药细胞BCL2基因表达增加,STAT3-BCL2表达被AURKB抑制剂高度抑制。然而,在H1993 PR-S2细胞中,STAT3或BCL2的敲除并未增强MET-TKI的敏感性。此外,在低浓度的AURKB抑制剂下,H1993 PR-S2细胞中切割-caspase3的表达升高,G2/M期阻滞。AURKB抑制剂对H1993 PR-S2肿瘤异种移植物也显示出较强的抗肿瘤活性。最后,我们证实了晚期met扩增肺癌患者对met靶向药物获得性耐药的治疗后肿瘤中AURKB和p-STAT3表达上调。这些发现表明,AURKB是met - tki耐药met扩增肺癌治疗的潜在药物靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Overcoming MET-targeted drug resistance in MET-amplified lung cancer by aurora kinase B inhibition
MET-targeted therapies are the most effective treatment for patients with MET-amplified lung cancer. However, acquired drug resistance is a significant challenge in MET-amplified lung cancer treatment. This study aimed to discover an effective treatment strategy for overcoming MET-targeted drug resistance. We first established a lung cancer cell line resistant to MET tyrosine kinase inhibitor (MET-TKI) (H1993 PR-S2) from MET-amplified lung cancer cells (H1993). High-throughput screening using an anti-cancer compound library identified Aurora Kinase B (AURKB) inhibitor as a potent agent suppressing H1993 PR-S2 cell viability. In these resistant cells, p-MET expression was markedly decreased, while p-AURKB was significantly increased. Furthermore, STAT3-activated gene signatures were enriched in H1993 PR-S2 cells, and p-STAT3 expression was closely linked to AURKB. The AURKB overexpression induced p-STAT3 activation in the parental cells, whereas the AURKB knockdown reduced p-STAT3 expression in the H1993 PR-S2 cells. The resistant cells showed increased BCL2 gene expression, and STAT3-BCL2 expression was highly suppressed by AURKB inhibitor. However, MET-TKI sensitivity was not enhanced by STAT3 or BCL2 knockdown in H1993 PR-S2 cells. Additionally, the elevated expression of cleavage-caspase3 and the G2/M phase arrest were observed at lower concentrations of AURKB inhibitor in the H1993 PR-S2 cells. AURKB inhibitor also showed potent anti-tumor activity against the H1993 PR-S2 tumor xenografts. Finally, we confirmed the upregulated AURKB and p-STAT3 expression in post-treatment tumors of advanced MET-amplified lung cancer patient who experienced acquired resistance to MET-targeted drugs. These findings suggest AURKB is a potential druggable target for MET-TKI-resistant MET-amplified lung cancer treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.00
自引率
2.00%
发文量
151
审稿时长
44 days
期刊介绍:
BBA Molecular Cell Research focuses on understanding the mechanisms of cellular processes at the molecular level. These include aspects of cellular signaling, signal transduction, cell cycle, apoptosis, intracellular trafficking, secretory and endocytic pathways, biogenesis of cell organelles, cytoskeletal structures, cellular interactions, cell/tissue differentiation and cellular enzymology. Also included are studies at the interface between Cell Biology and Biophysics which apply for example novel imaging methods for characterizing cellular processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


