parl介导的线粒体蛋白酶活性对钙调节的影响。
IF 3.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular cell research
Pub Date : 2025-06-06
DOI:10.1016/j.bbamcr.2025.119998
引用次数: 0
摘要
早老素相关菱形样蛋白(PARL)是一种线粒体内膜丝氨酸蛋白酶,是细胞凋亡、代谢、炎症和应激反应等细胞过程的关键调节因子。虽然最近的研究表明,PARL可能在线粒体钙稳态中起作用,但其潜在的机制仍然知之甚少。在这项研究中,我们研究了PARL调节对线粒体和细胞质钙动力学以及线粒体膜电位的影响。我们的研究结果表明,通过过表达和沉默改变PARL蛋白水平,显著影响线粒体钙摄取,而不影响细胞质钙瞬态或线粒体膜电位。尽管观察到线粒体钙动力学的变化,但PARL不与线粒体钙单转运复合物(mtCU)调节因子MICU1和MICU2相互作用,而MICU1和MICU2对调节线粒体钙内流至关重要。然而,我们观察到MICU1和MICU2的单体或二聚体形式的蛋白水平变化,这表明PARL可能间接影响这些mtCU成分。有趣的是,孔隙形成亚基MCU和结构亚基EMRE是mtCU组装所必需的,不受PARL调制的影响。这些发现表明,PARL在调节线粒体钙稳态中的作用可能涉及间接机制,可能涉及其他调节途径。总的来说,我们的研究为PARL在线粒体钙调节中的功能作用提供了新的见解,为进一步研究其更广泛的细胞功能提供了潜在的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
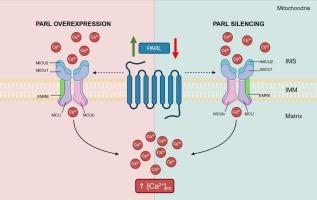
Impact of PARL-mediated mitochondrial protease activity on calcium regulation
The presenilin-associated rhomboid-like protein (PARL) is a mitochondrial inner membrane serine protease that is a key regulator of several cellular processes, including apoptosis, metabolism, inflammation and stress responses. While recent studies suggest that PARL may play a role in mitochondrial calcium homeostasis, the underlying mechanisms remain poorly understood. In this study, we investigated the effects of PARL modulation on mitochondrial and cytosolic calcium dynamics, as well as mitochondrial membrane potential. Our results show that altering PARL protein levels, through both overexpression and silencing, significantly affects mitochondrial calcium uptake, without influencing cytosolic calcium transients or mitochondrial membrane potential. Despite the observed changes in mitochondrial calcium dynamics, PARL does not interact with the mitochondrial calcium uniporter complex (mtCU) regulators MICU1 and MICU2, which are critical for regulating mitochondrial calcium influx. However, we observed alterations in the protein levels of MICU1 and MICU2, either in their monomeric or dimeric forms, suggesting that PARL may influence these mtCU components indirectly. Interestingly, the pore-forming subunit MCU, and the structural subunit EMRE, essential for the assembly of the mtCU, were unaffected by PARL modulation. These findings suggest that the role of PARL in modulating mitochondrial calcium homeostasis may involve indirect mechanisms, potentially involving other regulatory pathways. Overall, our study provides novel insights into the functional role of PARL in mitochondrial calcium regulation, offering potential avenues for further investigation into its broader cellular functions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.00
自引率
2.00%
发文量
151
审稿时长
44 days
期刊介绍:
BBA Molecular Cell Research focuses on understanding the mechanisms of cellular processes at the molecular level. These include aspects of cellular signaling, signal transduction, cell cycle, apoptosis, intracellular trafficking, secretory and endocytic pathways, biogenesis of cell organelles, cytoskeletal structures, cellular interactions, cell/tissue differentiation and cellular enzymology. Also included are studies at the interface between Cell Biology and Biophysics which apply for example novel imaging methods for characterizing cellular processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


