载PTH1-34肽的多孔PLGA微球用于OA的长期治疗
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
摘要
骨关节炎(OA)是一种以关节软骨退行性变为特征的慢性疾病,影响全球超过5.3亿患者。目前的口服药物,如非甾体抗炎药(NSAIDs)只能缓解症状,并伴有许多不良反应。虽然特利帕肽(teriparatide, PTH1-34)具有软骨保护和成骨作用的双重功能,但其生物半衰期短(30-60分钟),且在关节腔炎症微环境中降解加速,严重限制了其临床应用。方法以经fda批准的聚乳酸-羟基乙酸(PLGA)为基质制备多孔缓释微球(M@PTH1-34),将PTH1-34包埋在其多通道多孔结构中。通过膜乳化和温控包埋技术制备了均匀的微球,实现了高效载药。为了系统评价其缓释情况和治疗效果,我们建立了体外和体内OA模型,综合分析其软骨修复功效、抗炎调节和免疫调节作用。结果spth1 -34自愈后可有效加载微球,并在30 d内持续释放,保持生物活性。在OA模型大鼠中,M@PTH1-34显著改善了行为和影像学结果,增加了软骨平滑度和厚度,并增加了软骨生成标志物的表达。此外,体外和体内安全性试验未发现明显的安全性问题。这些发现表明M@PTH1-34有望成为一种持久、经济、安全的OA治疗方法。本研究成功开发了一种均匀大小的基于plga的缓释微球系统(M@PTH1-34),可以在单次关节内给药后持续释放药物30天以上。M@PTH1-34通过以下两种途径发挥其对骨关节炎的治疗作用:(1)通过增强骨髓间充质干细胞(BMSCs)的成软骨分化能力促进软骨修复;(2)通过抑制炎症因子(如IL-1β)的表达,调节M1/M2巨噬细胞的极化状态,改善关节炎症微环境。该系统具有突出的临床翻译优势:(1)创新利用fda批准的PLGA载体,结合膜乳化技术,保证了尺寸的精确控制和标准化生产;(2)局部给药策略在关节腔内实现了目标潴留,动物实验证实无系统性暴露风险;(3)标准化的制备工艺证明了工业化规模生产的可行性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
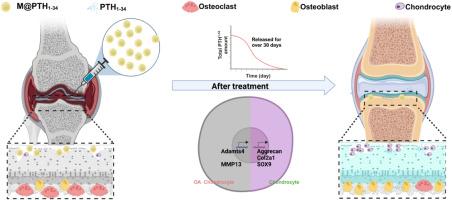
Porous PLGA microspheres loaded with PTH1-34 peptide for long-term treatment of OA
Background
Osteoarthritis (OA) is a chronic disease characterized by degeneration of articular cartilage, affecting over 530 million patients worldwide. Current oral medications such as non-steroidal anti-inflammatory drugs (NSAIDs) can only alleviate symptoms and are associated with numerous adverse effects. Although teriparatide (PTH1-34) exhibits dual functions of chondroprotection and osteogenic effects, its clinical application is significantly limited by its short biological half-life (30–60 min) and accelerated degradation within the inflammatory microenvironment of joint cavities.
Methods
Porous sustained-release microspheres (M@PTH1-34) were fabricated using FDA-approved poly (lactic-co-glycolic acid) (PLGA) as the matrix, encapsulating PTH1-34 within their multi-channel porous structure. Uniform microsphere preparation and high-efficiency drug loading were achieved through membrane emulsification and temperature-controlled embedding techniques. To systematically evaluate the sustained-release profile and therapeutic outcomes, both in vitro and in vivo OA models were established, enabling comprehensive analysis of cartilage repair efficacy, anti-inflammatory regulation, and immunomodulatory effects.
Results
PTH1-34 could be efficiently loaded into microspheres after self-healing and achieve consistent release over 30 days with biological activity being maintained. In OA model rats, M@PTH1-34 significantly improved behavioral and radiological outcomes, increased cartilage smoothness and thickness, and increased the expression of chondrogenic markers. Additionally, in vitro and in vivo safety tests revealed no significant safety issues. These findings indicate that M@PTH1-34 holds promise as a long-lasting, cost-effective, and safe therapeutic approach for OA.
Conclusion
This study successfully developed a uniform-sized PLGA-based sustained-release microsphere system (M@PTH1-34) that enables continuous drug release for over 30 days following single intra-articular administration. M@PTH1-34 exerts its therapeutic effects on osteoarthritis through the following two ways: (1) Promoting cartilage repair by enhancing the chondrogenic differentiation ability of bone marrow mesenchymal stem cells (BMSCs); (2) Improve the inflammatory microenvironment of joints by inhibiting the expression of inflammatory factors (such as IL-1β) and regulating the polarization state of M1/M2 macrophages.
The translation potential of this article
The system demonstrates prominent clinical translation advantages: (1) Innovative utilization of FDA-approved PLGA carrier combined with membrane emulsification technique ensures precise size control and standardized production; (2) Localized delivery strategy achieves targeted retention within articular cavity, validated by animal studies showing no systemic exposure risks; (3) Standardized preparation process demonstrates the feasibility of industrial-scale production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


