基于软骨细胞外基质微载体构建软骨类器官,通过免疫调节促进关节软骨再生
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
摘要
目的探讨软骨细胞外基质微载体(CEMMs)构建软骨类器官(CORGs)的可行性,评价其异位成软骨潜能,并分析其对SD大鼠膝关节软骨原位修复和再生的影响。方法采用脱细胞、湿磨和分层筛分相结合的方法制备软骨细胞外基质微载体。在实验室环境下,通过活/死染色、CCK-8法、划痕法和Transwell法评估其生物学功能。通过大鼠巨噬细胞的条件培养,证实了免疫微环境受到cemm的影响。采用qRT-PCR和分泌功能检测评价CORGs的成软骨活性。利用转录组测序分析了CORGs发育过程中的基因表达谱。免疫缺陷小鼠皮下模型评估CORGs异位软骨形成能力。将CORGs植入SD大鼠膝关节软骨缺损,观察其对软骨再生的影响。结果成功制备的尺寸为210.4±56.89 um的cemm具有较强的生物相容性,能够吸引干细胞,刺激其生长和迁移,并促进巨噬细胞向M2型转移。在cemm的基础上成功构建了功能化corg。转录组学显示,CORGs具有类似于中胚层到软骨发育的基因表达模式。CORGs成功地在免疫缺陷小鼠皮下生成软骨组织。具体而言,在术后1周,观察到CORGs促进关节周围巨噬细胞的M2极化。术后6周和12周,大体观察、显微ct扫描和组织学分析均显示CORGs促进软骨再生。结论在cemm的基础上成功构建了功能化的CORGs,显示出软骨相关基因的强烈表达,并显示出分泌胶原和gag的能力。转录组学分析显示,CORGs的基因表达轨迹与中胚层基因向软骨基因的转变一致,导致免疫缺陷小鼠皮下植入富含软骨特异性基质的软骨组织成功发育。此外,CORGs显示了调节膝关节周围免疫微环境的能力。在SD大鼠膝关节软骨缺损模型中,CORGs表现出强大的再生和修复能力。本研究涉及利用天然生物材料(ECM)和间充质干细胞创建CORGs,显示出治疗软骨损伤的重大前景,从而为软骨组织再生工程的创新策略铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
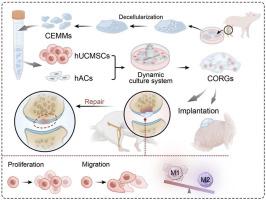
Construction of cartilaginous organoids based on cartilage extracellular matrix microcarriers to promote articular cartilage regeneration through immune regulation
Objective
To investigate the feasibility of constructing cartilaginous organoids (CORGs) using cartilage extracellular matrix microcarriers (CEMMs), evaluate their ectopic chondrogenic potential, and analyze their impact on in situ repair and regeneration of knee cartilage in SD rats.
Methods
Cartilage extracellular matrix microcarriers (CEMMs) were created through a combination of decellularization, wet milling, and layered sieving methods. The evaluation of their biological function was conducted through live/dead staining, CCK-8 assay, scratch assay, and Transwell assay in a laboratory setting. The immune microenvironment was confirmed to be influenced by CEMMs through a conditioned culture involving rat macrophages. qRT-PCR and secretory function assays was conducted to evaluate the chondrogenic activity of CORGs. Gene expression profiles throughout the development of CORGs were analyzed using transcriptome sequencing. Immunodeficient mouse subcutaneous model to assess the ectopic chondrogenic capacity of CORGs. CORGs were implanted into the knee joint cartilage defects of SD rats to evaluate their effects on cartilage regeneration.
Results
Successfully developed CEMMs with dimensions of 210.4 ± 56.89 um exhibited strong biocompatibility, the capacity to draw in stem cells, stimulate their growth and migration, and encourage macrophages to shift to the M2 type. Functionalized CORGs were successfully constructed based on CEMMs. Transcriptomics showed that CORGs had a gene expression pattern similar to mesodermal to chondrogenic development. CORGs successfully generated cartilaginous tissue subcutaneously in immunodeficient mice. Specifically, at 1 week postoperatively, CORGs were observed to promote M2 polarization of periarticular macrophages. At 6 and 12 weeks post-surgery, gross observation, micro-CT scanning, and histological analyses collectively revealed that CORGs promoted cartilage regeneration.
Conclusions
The functionalized CORGs was successfully constructed based on CEMMs, exhibiting robust expression of chondrogenic-related genes and demonstrating the ability to secrete collagen and GAGs. Transcriptomic analysis revealed that CORGs exhibited a gene expression trajectory consistent with the transition from mesodermal to chondrogenic genes, resulting in the successful development of cartilaginous tissues rich in cartilage-specific matrix when implanted subcutaneously in immunodeficient mice. Furthermore, CORGs demonstrated the ability to modulate the immune microenvironment surrounding the knee joint. In SD rat models of knee cartilage defects, CORGs exhibited robust regenerative and repair capacity.
The translational potential of this article
This research involved the creation of CORGs utilizing natural biomaterials (ECM) and MSCs, demonstrating significant promise for treating cartilage injuries, thereby paving the way for innovative strategies in cartilage tissue regeneration engineering.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


