低温钠离子电池的超快共插化学
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
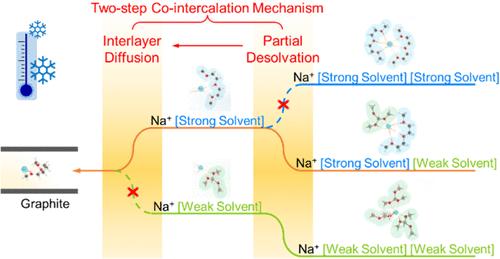
钠离子电池(sib)由于其低成本和丰富的元素资源,特别是在传统电池难以适应的寒冷环境中,为大规模储能提供了一个可持续和有前途的解决方案。石墨阳极的共插化学由于其快速的插层动力学而呈现出一条潜在的途径,但它在低温下面临着重大的挑战。在此,我们首先揭示了以前被忽视的共嵌层体系中的脱溶行为,这是低温下性能衰减的关键因素。我们提出了一种新的两步反应机制,包括部分脱溶和层间扩散的共插层化学,这表明了单溶剂溶剂化结构在实现整体动力学方面的挑战。在此基础上,我们开发了一种由溶剂组成的电解质,具有强溶剂化能力和弱溶剂化能力,可以加速上述两个动态过程。得益于独特的双溶剂溶剂化结构,弱溶剂化溶剂易于去除,实现了快速部分脱溶,强溶剂化Na+驱动层间快速扩散,固态核磁共振(ss-NMR)验证了这一点。与室温下的1℃相比,组装后的电池在- 30℃下的容量保持率高达~ 90.0%。在此温度下,当倍率从0.1℃增加到5℃时,电池仍然表现出优异的倍率性能,容量保持率高达~ 84%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ultrafast Cointercalation Chemistry for Low-Temperature Sodium-Ion Batteries
Sodium-ion batteries (SIBs) offer a sustainable and promising solution for large-scale energy storage because of their low cost and abundant element resources, especially in cold environments, where traditional batteries struggle. The cointercalation chemistry for graphite anode presents a potential avenue due to its fast intercalation kinetics, but it faces significant challenges at low temperatures. Herein, we first unravel a previously overlooked desolvation behavior in the cointercalation system, a key factor in performance decay under low temperatures. We propose a novel two-step reaction mechanism involving partial desolvation and interlayer diffusion for the cointercalation chemistry, which demonstrates the challenge of single-solvent solvation structures in achieving overall kinetics. Based on this, we developed an electrolyte composed of solvents with strong and weak solvation capabilities to accelerate the above two dynamic processes. Benefiting from the unique dual-solvent solvation structure, fast partial desolvation is realized by the easy removal of weakly solvating solvents, while rapid interlayer diffusion is driven by solvated Na+ with strong solvents, verified by solid-state nuclear magnetic resonance (ss-NMR). The assembled battery shows an ultrahigh capacity retention of up to ∼90.0% at −30 °C compared with that at room temperature at 1 C. Under this temperature, the battery still shows excellent rate performance with a high capacity maintenance of ∼ 84% for the rate increasing from 0.1 to 5 C.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


