序贯治疗调节炎症微环境和肌腱分化促进糖尿病大鼠肌腱愈合
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
摘要
慢性肌腱病变合并糖尿病(CTDM)由于持续的炎症和肌腱生成受损,给治疗带来了重大挑战。虽然补充肌腱干/祖细胞(TSPCs)有可能促进肌腱形成,但在炎症微环境中过早募集和增殖有纤维化或异位骨化(HO)的风险。因此,平衡炎症调节和肌腱分化是有效愈合的关键。方法以氧化透明质酸修饰多巴胺和苯硼酸功能化羧甲基壳聚糖为原料,制备葡萄糖反应性双药序贯给药水凝胶(GDSH)。将包封鸢尾素和结缔组织生长因子(CTGF)的树突状介孔二氧化硅纳米球(DMSNs)掺入GDSH基质中。对水凝胶的性能进行了全面的表征,包括流变性、机械性、粘附性、溶胀/降解和药物释放行为。体外评估评估细胞相容性,抗氧化和抗炎作用,以及TSPCs的迁移,增殖和分化。采用I型胶原酶/链脲佐菌素诱导的大鼠CTDM模型,通过组织学、生物力学和显微ct方法分析其治疗效果。转录组测序和Western blot分析被用来阐明特定信号通路在组织修复过程中的参与。结果GDSH复合水凝胶具有优异的机械强度、良好的黏附性、良好的生物相容性、适宜的溶胀和降解速率,以及可控的顺序释放能力。体外实验表明,这些复合水凝胶具有抗氧化和抗炎作用,同时还能促进细胞增殖和迁移。此外,它们促进了TSPCs的成腱分化,同时抑制了TSPCs的异常分化。体内研究表明,复合水凝胶可显著改善损伤肌腱的形态和生物力学特性,减少炎症,纠正异常分化,并具有良好的生物安全性。转录组测序和Western blotting分析表明,复合水凝胶通过MAPK、AMPK、Smad、Hippo和PI3K/AKT信号通路修复CTDM。结论dsh通过鸢尾素和CTGF的葡萄糖响应性顺序递送实现炎症消退和肌腱生成的时空控制。该策略恢复了CTDM中肌腱的微观结构、生物力学和氧化还原稳态,为糖尿病肌腱再生提供了可翻译的平台。本研究提出了一种葡萄糖反应性双药顺序递送水凝胶(GDSH),设计用于治疗慢性肌腱病变合并糖尿病(CTDM)。这种创新的方法旨在平衡炎症调节和促进肌腱分化。鸢尾素和结缔组织生长因子(CTGF)的顺序释放有效地解决了肌腱修复过程中氧化应激/炎症和异常分化带来的双重挑战。该水凝胶具有良好的生物相容性、药物释放控制和修复肌腱结构和功能的功效,具有临床应用潜力。该平台代表了一种比传统疗法更安全、更有效的替代方案。未来的研究应侧重于扩大生产规模,评估长期安全性,并促进将该技术转化为糖尿病患者肌腱损伤管理的人体临床试验。本文章由计算机程序翻译,如有差异,请以英文原文为准。
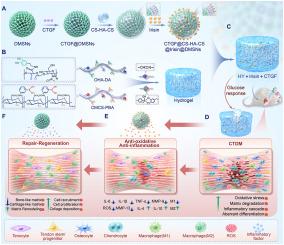
Regulating inflammation microenvironment and tenogenic differentiation as sequential therapy promotes tendon healing in diabetic rats
Background
Chronic tendinopathy with diabetes mellitus (CTDM) poses significant therapeutic challenges due to persistent inflammation and impaired tenogenesis. While the supplementation of tendon stem/progenitor cells (TSPCs) has the potential to facilitate tenogenesis, premature recruitment and proliferation in inflammatory microenvironments risks fibrosis or heterotopic ossification (HO). Consequently, balancing inflammation regulation and tenogenic differentiation is critical for effective healing.
Methods
An injectable glucose-responsive dual-drug-sequential delivery hydrogel (GDSH) was developed utilizing oxidized hyaluronic acid-modified dopamine and phenylboronic acid-functionalized carboxymethyl chitosan. Dendritic mesoporous silica nanospheres (DMSNs) encapsulating irisin and connective tissue growth factor (CTGF) were incorporated into the GDSH matrix. A comprehensive characterization of the hydrogel's properties, including rheological, mechanical, adhesive, swelling/degradation, and drug release behaviors, was conducted. In vitro assessments were performed to evaluate cytocompatibility, as well as antioxidant and anti-inflammatory effects, alongside the migration, proliferation, and differentiation of TSPCs. The therapeutic efficacy was further investigated using a collagenase type I/streptozotocin-induced CTDM model in rats, with analyses conducted through histological, biomechanical, and micro-CT methods. Transcriptome sequencing and Western blot analyses were employed to elucidate the involvement of specific signaling pathways in the tissue repair process.
Results
The GDSH composite hydrogels possess a range of advantageous properties, including exceptional mechanical strength, optimal adhesiveness, superior biocompatibility, and appropriate swelling and degradation rates, in addition to controllable and sequential drug release capabilities. In vitro investigations revealed that these composite hydrogels exhibit antioxidant and anti-inflammatory effects, while also promoting cell proliferation and migration. Furthermore, they facilitate tenogenic differentiation and simultaneously inhibit the aberrant differentiation of TSPCs. In vivo studies demonstrated that the composite hydrogels significantly improved the morphological and biomechanical properties of injured tendons, reduced inflammation, corrected abnormal differentiation, and displayed favorable biosafety profiles. Transcriptome sequencing and Western blotting analysis indicated that the composite hydrogels repaired CTDM through the MAPK, AMPK, Smad, Hippo and PI3K/AKT signaling pathways.
Conclusion
GDSH achieves spatiotemporal control of inflammation resolution and tenogenesis via glucose-responsive sequential delivery of irisin and CTGF. This strategy restores tendon microstructure, biomechanics, and redox homeostasis in CTDM, offering a translatable platform for diabetic tendon regeneration.
The Translational Potential of this Article
This study presents a glucose-responsive dual-drug-sequential delivery hydrogel (GDSH) designed for the treatment of chronic tendinopathy with diabetes mellitus (CTDM). This innovative approach aims to balance the regulation of inflammation and promote tenogenic differentiation. The sequential release of irisin and connective tissue growth factor (CTGF) effectively addresses the dual challenges posed by oxidative stress/inflammation and aberrant differentiation during tendon repair. The hydrogel's demonstrated biocompatibility, controlled drug release, and efficacy in restoring tendon structure and function highlight its potential for clinical translation. This platform represents a safer and more effective alternative to conventional treatments. Future research should focus on scaling up production, assessing long-term safety, and facilitating the translation of this technology into human clinical trials for the management of tendon injuries in diabetic patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


