微量反应中氨基烷基自由基诱导的卤素原子转移
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
卤素原子转移(XAT)为烷基卤化物转化为相应的碳自由基提供了一种可行的策略,但通常不可避免地要使用等效的XAT试剂和过量的氧化剂。在此,我们提出了一个无迹氨基烷基自由基诱导的Minisci反应的XAT过程,特别是在氧化还原中性条件下没有过量XAT试剂的参与。机理实验表明,类似的氨基烷基自由基的形成是通过质子化杂芳烃的单电子转移还原发生的,与传统的胺氧化生成α-氨基烷基自由基的过程不同。本文章由计算机程序翻译,如有差异,请以英文原文为准。
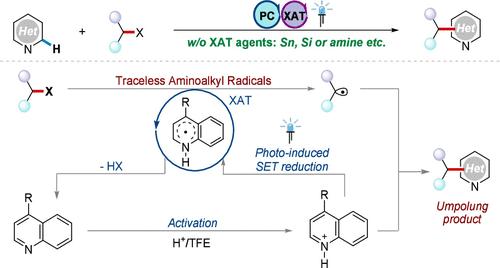
Traceless Aminoalkyl Radical-Induced Halogen-Atom Transfer for Minisci Reactions
Halogen-atom transfer (XAT) provides a viable strategy to convert alkyl halides into the corresponding carbon radicals, but the usage of equivalent XAT reagents and excess oxidants is usually inevitable. Herein, we present a traceless aminoalkyl radical-induced XAT process for the Minisci reaction, especially without the participation of excess XAT reagents under redox-neutral conditions. Mechanistic experiments indicated that the formation of comparable aminoalkyl radicals occurred through the single-electron transfer reduction of protonated heteroaromatics, differing from the conventional oxidation process of amines used to generate α-aminoalkyl radicals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


