光氧化还原自催化作用下α-酮酸的直接碳同位素交换
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
研究人员开发了一种光氧化还原自催化策略,利用标记的CO2直接交换α-酮酸的碳同位素,而不需要化学计量羧酸活化或外部光催化剂。机理研究表明,低能垒下C-CO2键的有效裂解是由羧酸盐单电子氧化触发的,随后是自由基-极性交叉过程,产生酰基阴离子,然后与标记的CO2重新结合。本文章由计算机程序翻译,如有差异,请以英文原文为准。
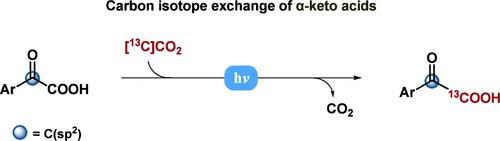
Direct Carbon Isotope Exchange of α-Keto Acids Enabled by Photoredox Self-Catalysis
A photoredox self-catalysis strategy has been developed for the direct carbon isotope exchange of α-keto acids using labeled CO2, bypassing the need for stoichiometric carboxylate activation or external photocatalysts. Mechanistic studies reveal that efficient cleavage of the C–CO2 bond at low energy barriers is triggered by carboxylate single-electron oxidation, followed by a radical-polar crossover process that generates acyl anions, which then recombine with the labeled CO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


