合成n -烷基和n -芳基异恶唑烷的分子内N-O键形成
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
我们描述了一系列易于组装的、不同取代的3-(4-三氟甲基-2-硝基苯磺酰胺)烷基硅基过氧化物的合成,并通过去除磺胺保护基团形成分子内N-O键,以中高收率转化为相应的异恶唑烷。N-O键形成的环化依赖于亲电性硅基过氧化物的位阻,在磺胺与硫代阴离子裂解过程中,初级体系直接环化。另一方面,二级硅基过氧化物在脱硫后最好在六氟异丙醇存在下通过加热进行环化,而三级硅基过氧化物则需要中间胺的去质子化。在氟化物介导的脱硅过程中,叔苯基过氧化物以一种新机制环化,形成中间的三氟甲基硝基苯过氧化物。环化扩展到包括一个简单的恶氮杂的形成,但外推到形成一个恶氮杂被竞争Kornblum DeLaMare碎片在中间氨基过氧化物的水平被阻止。本文章由计算机程序翻译,如有差异,请以英文原文为准。
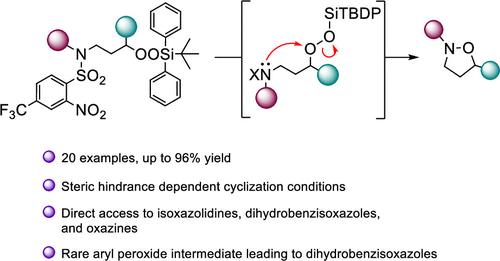
Intramolecular N–O Bond Formation for the Synthesis of N-Alkyl and N-Aryl Isoxazolidines
We describe the synthesis of a series of readily assembled, variously substituted 3-(4-trifluoromethyl-2-nitrobenzenesulfonamido)alkyl silylperoxides and their conversion to the corresponding isoxazolidines in moderate to high yield by intramolecular N–O bond formation on removal of the sulfonamide protecting group. Cyclization with N–O bond formation was dependent on steric hindrance of the electrophilic silylperoxides, with primary systems cyclizing directly during the course of sulfonamide cleavage with thiolate anions. Secondary silyl peroxides, on the other hand, were best cyclized by warming in the presence of hexafluoroisopropanol after desulfonylation, while tertiary silylperoxides required deprotonation of the intermediate amine. Tertiary benzylic peroxides underwent cyclization by a novel mechanism, with the formation of intermediate trifluoromethylnitrophenyl peroxides, during the course of fluoride-mediated desilylation. The cyclization was extended to include the formation of a simple oxazine, but extrapolation to the formation of an oxazepine was foiled by competing Kornblum DeLaMare fragmentation at the level of the intermediate aminoperoxide.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


