基于串联的双分子催化剂,可实现电催化CO2 -甲醛-甲醇级联转化
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
电催化二氧化碳还原成多电子产物是一种很有前途的碳捕获和利用方法。近年来,以酞菁钴(CoPc)为基础的分子催化剂在电化学将CO2转化为甲醇(一种6e−/6H+产物)方面表现出了潜在的能力。然而,尽管最近取得了进展,但CoPc的聚集倾向和弱co中间体结合通常限制了其电催化活性和选择性。在此,我们证明了金属-有机框架(MOF)可以通过固定两种分子催化剂(CoPc和fe -卟啉)来构建串联电催化体系。值得注意的是,与仅使用copc的mof基催化剂相比,mof基串联催化剂的二氧化碳-甲醇电催化活性和选择性提高了3倍(在25 mA/cm2时,甲醇法拉第效率高达18%)。此外,operando光谱和电化学分析表明,与典型的串联系统不同,基于mof的串联系统通过使用不同于CO的反应中间体(即甲醛)来独特地工作。因此,这种概念验证方法为设计能够驱动复杂质子耦合电子转移反应的分子电催化方案提供了一种新方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
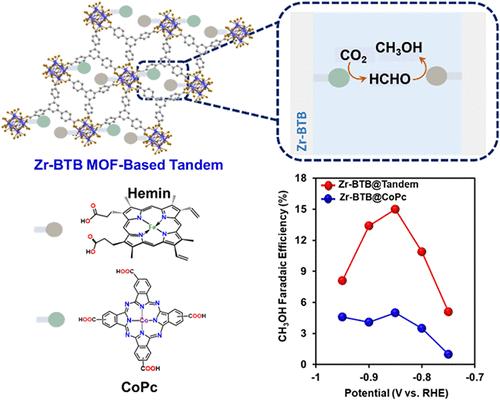
Dual Molecular Catalyst-Based Tandem That Enables Electrocatalytic CO2−Formaldehyde−Methanol Cascade Conversion
Electrocatalytic CO2 reduction into multielectron products is a promising approach for carbon capture and utilization. Recently, cobalt phthalocyanine (CoPc)-based molecular catalysts have shown potential competence toward electrochemical conversion of CO2 to methanol, a 6e−/6H+ product. Yet, despite the recent advancements, CoPc’s tendency to aggregate and the weak CO-intermediate binding generally limit its electrocatalytic activity and selectivity. Herein, we demonstrate that a metal−organic framework (MOF) could be used to construct a tandem electrocatalytic system via immobilization of 2 types of molecular catalysts (CoPc and Fe-porphyrin). Notably, the MOF-based tandem achieves a 3-fold increase in electrocatalytic CO2-to-methanol activity and selectivity compared to a CoPc-only MOF-based catalyst (up to 18% methanol faradaic efficiency at 25 mA/cm2). Additionally, operando spectroscopy and electrochemical analysis show that unlike typical tandem systems, the MOF-based tandem operates uniquely by using a reactive intermediate different from CO (i.e., formaldehyde). Hence, this proof-of-concept approach offers a new means to design molecular electrocatalytic schemes capable of driving complex proton-coupled electron transfer reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


