镍催化二芳基二硫化物与芳基溴的还原交叉偶联,通过C-S键裂解合成联芳基
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-09-05
DOI:10.1039/d5qo00546a
引用次数: 0
摘要
在以往的报道中,在过渡金属催化剂(如Ni、Cu)和金属介质(如Mg、Zn)的存在下,二芳基二硫化物与芳基卤化物的交叉偶联通常通过旧S-S键断裂和新的C-S键形成而产生相应的芳基硫化物。在本研究中,我们发现,在室温下,在镍催化剂、镁和氯化锂的存在下,二芳基二硫化物与芳基溴化物的还原交叉偶联通过异常的C-S键裂解进行,导致各种双芳基在中等到良好的产量。此外,该反应可以很容易地按比例放大。机理研究表明,该反应可能是通过原位生产芳基镁化合物作为反应中间体,通过licl促进Mg插入芳基溴而进行的。最重要的是,Ni(II)催化剂和Mg介质的组合使用是导致二芳基二硫化物中C-S键异常断裂形成相应的芳基镍(II)物质的关键,而芳基镍(II)物质是本脱硫交叉偶联反应的另一个重要中间体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
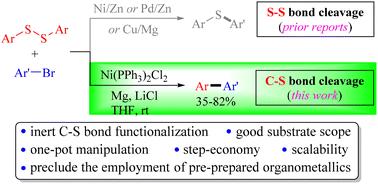
Ni-catalyzed reductive cross-couplings of diaryl disulfides with aryl bromides for biaryl synthesis through C–S bond cleavage†
In previous reports, the cross-couplings of diaryl disulfides with aryl halides in the presence of transition metal catalysts (e.g., Ni and Cu) and metal mediators (e.g., Mg and Zn) usually led to the corresponding aryl sulfides via old S–S bond cleavage and new C–S bond formation. In the present study, we found that the reductive cross-couplings of diaryl disulfides with aryl bromides proceeded via unusual C–S bond cleavage in the presence of a nickel catalyst, magnesium, and lithium chloride in THF at room temperature, leading to a variety of biaryls in modest to good yields. In addition, the reaction could be scaled up with ease. Mechanistic studies showed that the reaction possibly proceeds via the in situ production of an arylmagnesium compound as a reaction intermediate via LiCl-facilitated Mg insertion into aryl bromide. Most importantly, the combinatory use of the Ni(ii) catalyst and Mg mediator is key to the unusual cleavage of the C–S bond in diaryl disulfide to form the corresponding arylnickel(ii) species, which serves as another important intermediate in the present desulfurative cross-coupling reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


