一种扭曲多环芳烃衍生物的简易合成
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报道了一种简单的自下而上合成扭曲多环芳烃(PAH)衍生物。该结构具有三个扩展的七方环,由三环庚烯核心的结构延伸,并将三个蒽醌单元整合到π共轭结构中。这种策略使克级合成在温和条件下具有良好的产率。单晶x射线晶体学分析证实了由三个七面体引起的鞍形几何形状。这项工作为设计具有可调谐光电功能的高弯曲多环芳烃提供了一种通用策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
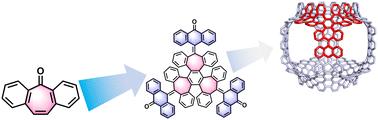
Facile synthesis of a contorted polycyclic aromatic hydrocarbon derivative†
A facile bottom-up synthesis of a contorted polycyclic aromatic hydrocarbon (PAH) derivative is reported. The structure features three expanded heptagonal rings, constructed by structural extension of a tris-cycloheptenylene core with the integration of three anthraquinone units into the π-conjugated architecture. This strategy enables gram-scale synthesis with decent yields under mild conditions. Single crystal X-ray crystallographic analysis confirmed a saddle-shaped geometry induced by the three heptagons. This work provides a generalizable strategy for designing highly curved PAHs with tunable optoelectronic functionalities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


