肠道类器官及其细胞外囊泡联合治疗炎症性肠病合并骨质疏松症
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
摘要
背景:炎症性肠病(IBD)伴骨质疏松症(OP)具有显著的临床合并症,目前尚无有效的治疗方法。通过三维(3D)共培养系统设计的肠道类器官(IOs)显示出内在的再生潜力。此外,来自IOs (ioev)的细胞外囊泡已被确定为能够调节肠道炎症的有效纳米介质。方法在本研究中,我们成功建立了io,并分离了ioev。ioev中的miRNA测序揭示了ibd相关的miRNA,这些miRNA可能减轻炎症反应并具有成骨作用。采用脂多糖(LPS)诱导炎症,建立IBD体外模型。此外,采用葡聚糖硫酸钠(DSS)诱导的IBD小鼠模型来评估其体内作用。结果在lps诱导的体外模型中,IOs和ioev均可减少细胞坏死和凋亡。在dss诱导的IBD小鼠模型中,治疗导致体重和结肠形态的恢复。组织学检查显示肠隐窝增加,组织结构正常化。免疫分析显示ZO-1和Ki67上调,Caspase-3下调,表明粘膜屏障完整性和细胞增殖增强,细胞凋亡减少。细胞因子谱显示促炎细胞因子TNF-α、IL-1β、IL-6下调,抗炎细胞因子IL-10上调。重要的是,IOs和ioev的联合应用逆转了IBD骨质疏松症的进展,改善了骨量和质量。总之,这些多模式的发现为通过类器官衍生的生物制剂调节肠道-骨轴建立了一个新的范例,为治疗ibd相关骨质疏松症提供了一个有希望的治疗策略。该研究强调了肠道类器官及其细胞外囊泡作为一种双作用生物疗法的转化潜力,可以缓解ibd相关骨质疏松症的肠道炎症和逆转骨质流失。ioev中功能性mirna的鉴定支持其作为治疗炎症性疾病全身性并发症的微创、无细胞疗法的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
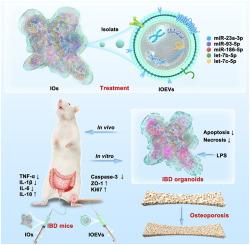
Combination therapy using intestinal organoids and their extracellular vesicles for inflammatory bowel disease complicated with osteoporosis
Background
Inflammatory bowel disease (IBD) with osteoporosis (OP) exhibits a clinically significant comorbidity, for which no effective treatment is currently available. Intestinal organoids (IOs), engineered through three-dimensional (3D) coculture systems, demonstrated intrinsic regenerative potentials. Additionally, extracellular vesicles derived from IOs (IOEVs) have been identified as potent nanoscale mediators capable of modulating intestinal inflammation.
Methods
In this study, we successfully established IOs and isolated IOEVs. miRNA sequencing in IOEVs revealed IBD-associated miRNAs, which may alleviate inflammatory response and have osteogenic effects. An in vitro model of IBD was established using lipopolysaccharide (LPS) to induce inflammation. Additionally, the dextran sulfate sodium (DSS)-induced IBD mouse model was employed to evaluate in vivo effects.
Results
In the LPS-induced in vitro model, treatment with IOs and IOEVs resulted in reduced cell necrosis and apoptosis. In DSS-induced IBD mouse models, treatment led to restoration of body weight and colon morphology. Histological assessment revealed an increase in intestinal crypts and normalization of tissue architecture. Immunological analyses showed upregulation of ZO-1 and Ki67 and downregulation of Caspase-3, suggesting enhanced mucosal barrier integrity and cellular proliferation with decreased apoptosis. Cytokine profiling showed downregulation of pro-inflammatory cytokines TNF-α, IL-1β, IL-6 and upregulation of anti-inflammatory cytokine IL-10. Importantly, the combination of IOs and IOEVs reversed osteoporosis progression in IBD, improving bone mass and quality.
Conclusion
Collectively, these multimodal findings establish a novel paradigm for gut–bone axis modulation through organoid-derived biologics, offering a promising therapeutic strategy for managing IBD-associated osteoporosis.
The translational potential of this article
This study highlights the translational potential of intestinal organoids and their extracellular vesicles as a dual-action biologic therapy that alleviates intestinal inflammation and reverses bone loss in IBD-associated osteoporosis. The identification of functional miRNAs within IOEVs supports their development as minimally invasive, cell-free therapeutics for systemic complications in inflammatory disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


