低温下固体电解质LiTi2(PS4)3的超快锂离子动力学
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
小原子或离子的自扩散过程在许多研究领域起着至关重要的作用。lit2 (PS4)3 (LTPS)独特的晶体结构为Li+小载流子提供了多种能量不等效扩散途径,并导致Li+扩散系数最高。在原子尺度上单独研究这些途径提出了重大挑战,特别是探测跳跃过程。在这项研究中,我们利用低至低温(10 K)的核自旋弛豫技术,揭示了Li+远程和短程动力学的前所未有的细节。温度相关的7Li核磁共振自旋-晶格弛豫速率(SLR)表现出一系列扩散诱导的峰,允许提取活化能和跳变速率。由于LTPS中异常快速的局部Li+交换过程,在SLR时间尺度上,需要低至50 K的温度来冻结Li+动力学,完全在LTPS结构的环形笼内。本文章由计算机程序翻译,如有差异,请以英文原文为准。
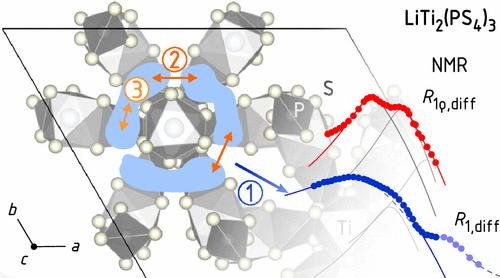
Unraveling Ultrafast Li-Ion Dynamics in the Solid Electrolyte LiTi2(PS4)3 by NMR down to Cryogenic Temperatures
Self-diffusion processes of small atoms or ions play a crucial role in many areas of research. The unique crystal structure of LiTi2(PS4)3 (LTPS) presents a variety of energetically inequivalent diffusion pathways for small Li+ charge carriers and has resulted in one of the highest Li+ diffusion coefficients. Investigating these pathways individually at the atomic scale poses significant challenges, especially for probing jump processes. In this study, we utilized nuclear spin relaxation techniques down to cryogenic temperatures (10 K) to reveal unprecedented details about both long-range and short-range Li+ dynamics. The temperature-dependent 7Li NMR spin–lattice relaxation (SLR) rate exhibits a series of diffusion-induced peaks, allowing the extraction of activation energies and jump rates. Due to the exceptionally fast localized Li+ exchange processes in LTPS, temperatures as low as 50 K are required to freeze Li+ dynamics, on the SLR time scale, entirely within the ring-like cages of the LTPS structure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


