来自洋葱伯克霍尔德菌复合体的有效抗真菌脂肽的复杂非核糖体组装
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
洋葱伯克霍尔德菌复合体(BCC)是一组革兰氏阴性菌,以其对囊性纤维化(CF)患者的致病性而闻名。bc属菌株B. pyrocinia BC11(原B. cepacia BC11)产生一种对植物病原真菌具有强活性的化合物AFC-BC11。在这篇文章中,我们报道了这种天然产物前所未有的n -酰基四肽结构和抗真菌效力。我们进一步提供的见解,其生物合成的中心步骤介导的非经典的非核糖体肽合成机制缺乏凝聚域。由于单一的酰基/肽基载体蛋白AfcK、酰基转移酶AfcL和辅酶a的参与,在复杂的生物合成组装过程中,生长的酰基肽链在不同的硫酯载体之间进行了重组。对afc - bc11结构的了解可能有助于开发抗植物病原体的抗真菌药物,并且由于afc基因簇在各种伯克霍尔德菌菌株中都是保守的,因此可能有助于了解人类BCC的发病机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
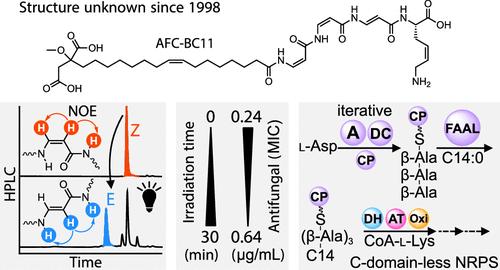
The Intricate Nonribosomal Assembly of a Potent Antifungal Lipopeptide from the Burkholderia cepacia Complex
The Burkholderia cepacia complex (BCC) is a group of Gram-negative bacteria known for their pathogenicity to patients suffering from cystic fibrosis (CF). The BCC-belonging strain B. pyrrocinia BC11 (formerly B. cepacia BC11) produces AFC-BC11, a compound with strong activity against phytopathogenic fungi. In this contribution, we report on the unprecedented N-acyl-tetrapeptide structure and antifungal potency of this natural product. We further provide insights into central steps of its biosynthesis mediated by a nonclassical nonribosomal peptide synthesis machinery lacking condensation domains. With the involvement of a sole acyl/peptidyl carrier protein AfcK, an acyltransferase AfcL and coenzyme A, the growing acyl-peptide chain is shuffled between different thioester carriers during the intricate biosynthetic assembly. The knowledge of the AFC-BC11 structure may contribute to the development of antifungals against phytopathogens and, with the afc gene cluster being conserved in various Burkholderia strains, possibly to an understanding of the human pathogenesis of the BCC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


