母体苝的合成及其衍生化
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们已经建立了一种实用且可扩展的(> 1g)合成苝,二苯并[cd,lm]苝的方法,从易于获得的2,3-二氢- 1h -phenalen-1- 1开始,首先通过还原偶联反应形成由26个碳原子组成的四氢-双苯二烯骨架,然后进行氧化光环化反应得到四氢-苝,最后进行芳构化。铱催化的对苝的直接硼化反应不具有完全的区域选择性,即使在控制试剂用量的情况下,也会产生由单硼化到六硼化衍生物组成的混合物。相反,四氢或八氢苝被证明是区域选择性硼化反应的合适底物,分别得到2,9-二硼化或2-(单)硼化苝。这些硼化的苝被用于合成各种苝衍生物,包括2,9-二酰基苝、二聚体苝和基于苝的大环,这表明硼化的苝是基于苝的光电材料的潜在中间体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
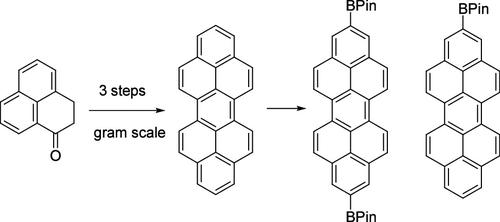
Synthesis of Parent Peropyrene and Its Derivatization
We have established a practical and scalable (>1 g) synthesis of peropyrene, dibenzo[cd,lm]perylene, from easily accessible 2,3-dihydro-1H-phenalen-1-one, which was first dimerized via the reductive coupling reaction to form the tetrahydro-biphenalenylidene skeleton consisting of 26 carbon atoms, followed by the oxidative photocyclization reaction giving tetrahydroperopyrene and the final aromatization. The iridium-catalyzed direct borylation reaction on peropyrene was not quite regioselective, giving a mixture consisting of mono- to hexaborylated derivatives even with a controlled amount of the reagents used. Instead, tetrahydro- or octahydroperopyrene turned out to be a suitable substrate for the regioselective borylation to give 2,9-diborylated or 2-(mono)borylated peropyrene, respectively. These borylated peropyrenes were utilized for the synthesis of various peropyrene derivatives, including 2,9-diarylperopyrenes, dimeric peropyrenes, and a peropyrene-based macrocycle, indicating that the borylated peropyrenes are potential intermediates for peropyrene-based optoelectronic materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


