肿瘤分泌的TNF通过TNFR1/NF-κB信号传导诱导乳腺癌相关脂肪细胞NCoA3上调
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-05-28
DOI:10.1016/j.bbadis.2025.167931
引用次数: 0
摘要
在乳腺癌中,脂肪细胞是微环境中的主要细胞类型,这些组织之间的持续交流改变了脂肪表型。然而,促进这些变化的分子机制仍然知之甚少。先前,我们证明NCoA3在乳腺癌附近的脂肪组织中表达增加,并且这种增加与炎症相关。本研究旨在探讨乳腺肿瘤微环境中脂肪细胞中NCoA3表达的机制。我们证明乳腺癌分泌的TNF增加了脂肪细胞中NCoA3的表达,这种上调依赖于NF-κB的转录活性。此外,TNF阻滞剂的使用阻止了共激活因子的过表达和巨噬细胞的募集,模拟了使用短发夹RNA下调NCoA3表达时观察到的效果。这些发现揭示了乳腺癌细胞调节脂肪细胞行为的分子机制,确定了NCoA3是肿瘤-脂肪组织串扰的关键介质。通过TNF抑制靶向这一途径为减轻肿瘤相关炎症提供了有希望的治疗策略,并可能改善乳腺癌患者的预后。本文章由计算机程序翻译,如有差异,请以英文原文为准。
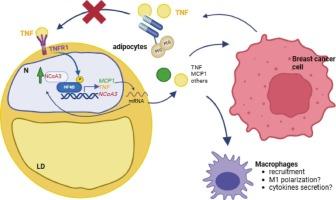
Tumor-secreted TNF induces NCoA3 upregulation in breast cancer-associated adipocytes via TNFR1/NF-κB signaling
In breast cancer, adipocytes are the predominant cell type in the microenvironment, and the continuous communication between these tissues alters the adipose phenotype. However, molecular mechanisms promoting these changes are still poorly understood. Previously, we demonstrated that NCoA3 expression is increased in adipose tissue adjacent to breast cancer and that this increase is associated with an inflammatory profile. This study aimed to investigate the mechanisms underlying NCoA3 expression in adipocytes within the breast tumor microenvironment.
We demonstrated that breast cancer-secreted TNF increases NCoA3 expression in adipocytes, and this upregulation is dependent on NF-κB transcriptional activity. Furthermore, the use of a TNF blocker prevented both coactivator overexpression and macrophages recruitment, mimicking the effects observed when NCoA3 expression was downregulated using a short hairpin RNA.
These findings shed light on the molecular mechanisms by which breast cancer cells modulate adipocyte behavior, identifying NCoA3 as a key mediator in the tumor-adipose tissue crosstalk. Targeting this pathway through TNF inhibition offers promising therapeutic strategy to attenuate tumor-associated inflammation and potentially improve outcomes in breast cancer patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


