通过脱对称控制二烷基卤化铵高压材料中烃类链熔化转变
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
含有碳氢化合物双分子层的层状材料能够在有序状态和部分无序状态之间转换,随着静水压力的变化,它们会表现出较大的温度和熵变化──称为气压效应。原则上,这些高压材料可用于驱动加热和冷却循环,比传统的氟碳制冷剂效率更高,对环境的影响更小。然而,为了设计具有特定热应用特性的材料,如何操纵碳氢化合物的有序-无序或“链熔化”相变的热力学和动力学仍有待了解。在这里,我们报道了一种链去对称策略来调节一种新的不对称二烷基卤化铵盐族的相变行为。特别是,我们证明了链不对称可以减少相变热滞后,同时保持大的熵变。这意味着可逆驱动非零熵变化所需的压力显著降低,不对称二烷基铵盐能够在比对称盐低近80%的压力下实现可逆熵变化。这项工作扩大了表现出强压热效应的链熔材料的范围,并提供了对影响压热材料可逆性的因素的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
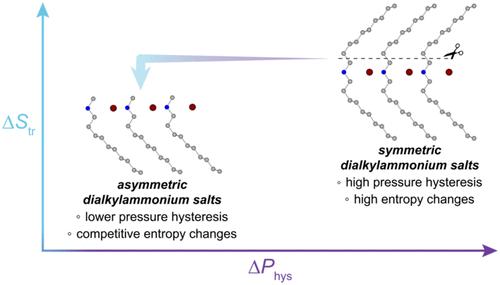
Manipulating Hydrocarbon Chain-Melting Transitions in Dialkylammonium Halide Barocaloric Materials through Desymmetrization
Layered materials containing hydrocarbon bilayers capable of transitioning between an ordered and partially disordered state can exhibit large temperature and entropy changes─termed barocaloric effects─in response to a change in hydrostatic pressure. These barocaloric materials can, in principle, be used to drive heating and cooling cycles with higher efficiency and less environmental impact than conventional fluorocarbon refrigerants. However, much remains to be understood about how to manipulate the thermodynamics and kinetics of hydrocarbon order–disorder, or “chain-melting”, transitions in the solid state in order to design materials with properties tailored for specific thermal applications. Here, we report a chain desymmetrization strategy to modulate the phase-change behavior of a new family of asymmetric dialkylammonium halide salts. In particular, we demonstrate that chain desymmetrization can lead to reduced phase-change thermal hysteresis while maintaining large entropy changes. This translates to a significant reduction in the pressure required to reversibly drive nonzero entropy changes, with asymmetric dialkylammonium salts able to access reversible entropy changes at pressures nearly 80% lower than their symmetric counterparts. This work expands the scope of chain-melting materials that exhibit strong barocaloric effects and offers insights into the factors that influence the reversibility of barocaloric materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


