鼠碱型生物碱的偶极环加成法:合成及立体化学测定
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过1,3-偶极环加成和易环重构,包括甲酰化、N-O键裂解和随后的环化,实现了毒草胺的快速合成。采用酶动力学拆分法得到光学富集的四氢β-羰基胺,并进一步细化制备毒胺。此外,还实现了有害生物酸的非对映异构体合成,并首次通过化学分辨和电子圆二色性计算实现了立体化学测定,为未来的药物研究提供了一个有趣的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
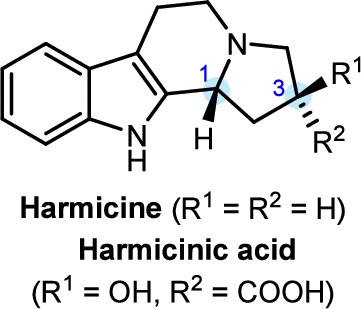
Dipolar Cycloaddition Approach toward Harmicine-Type Alkaloids: Synthesis and Stereochemical Determination
A rapid synthesis of harmicine was achieved through 1,3-dipolar cycloaddition and facile ring reconstruction, including mesylation, cleavage of the N–O bond, and subsequent cyclization. An enzymatic kinetic resolution was developed to obtain optically enriched tetrahydro-β-carboline, which was further elaborated to prepare harmicine. Additionally, diastereomeric synthesis of harmicinic acid was also achieved, and stereochemical determination was enabled by chemical resolution and electronic circular dichroism calculations for the first time, providing an intriguing platform to access various derivatives for future medicinal research.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


