纳米等离子体等吸揭示了金纳米颗粒在软模板上的中尺度组装
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
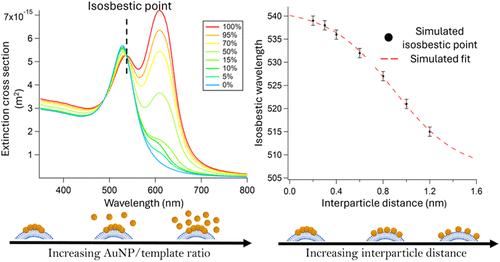
等离子体纳米粒子(NPs)的组装通过单个纳米粒子的局部表面等离子体共振(LSPR)耦合产生独特的光学特性。然而,精确控制和监测中尺度组装如何决定最终光学性质仍然是设计先进等离子体材料的关键挑战。在这里,我们引入了“纳米等离子体等吸”作为软模板上金纳米颗粒(AuNPs)中尺度组织的光学描述符。与描述化学平衡的分子光谱学中的等吸点不同,我们的数值模拟表明,纳米等离子体等吸来自于单个AuNP和AuNP簇的共存,其中粒子间距决定了等吸波长。通过模板化AuNP组装到具有可调膜刚度的合成独立脂质双层上,我们在实验上实现了对颗粒间距的精确控制,并证明了它是由等吸波长的单一调制反映的。这提供了对等离子体系统结构-功能关系的基本理解,首次将纳米等离子体等吸声与等离子体组件中的粒子间距和平衡结构联系起来。从分析的角度来看,纳米等离子体等吸提供了模板的非侵入性光学指纹,打开了吸引人的应用。作为概念证明,我们应用这种方法来分析两类细胞外囊泡(ev)的刚度─间充质干细胞(MSC)衍生和红细胞衍生的ev─两者都具有公认的生物学和翻译潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nanoplasmonic Isosbestics Uncover Mesoscale Assembly of Gold Nanoparticles on Soft Templates
Assembly of plasmonic nanoparticles (NPs) generates unique optical properties through coupling of the localized surface plasmon resonance (LSPR) of individual NPs. However, precisely controlling and monitoring how mesoscale assembly dictates final optical properties remain key challenges in designing advanced plasmonic materials. Here, we introduce “nanoplasmonic isosbestics” as optical descriptors of the mesoscale organization of gold nanoparticles (AuNPs) on soft templates. Unlike isosbestic points in molecular spectroscopy, which describe chemical equilibria, our numerical simulations demonstrate that nanoplasmonic isosbestics emerge from the coexistence of individual AuNPs and AuNP clusters, where the interparticle spacing determines the isosbestic wavelength. By templating AuNP assembly onto synthetic free-standing lipid bilayers with tunable membrane rigidity, we experimentally achieve precise control over interparticle spacing and prove that it is mirrored by univocal modulation of the isosbestic wavelength. This provides a fundamental understanding of the structure–function relationship in plasmonic systems, linking, for the first time, nanoplasmonic isosbestics to interparticle spacing and equilibrium structure in plasmonic assemblies. On the analytical perspective, nanoplasmonic isosbestics provide noninvasive optical fingerprints of the templates, opening to appealing applications. As a proof of concept, we apply this approach to profile the stiffness of two extracellular vesicle (EVs) classes─mesenchymal stem cell (MSC)-derived and red blood cell-derived EVs─both recognized for their biological and translational potential.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


