FTO在癌症发病、免疫逃避、化疗耐药和免疫治疗反应中的机制作用
IF 2.5
3区 医学
Q2 ONCOLOGY
引用次数: 0
摘要
脂肪质量和肥胖相关蛋白(FTO)是一种n6 -甲基腺苷(m6A) RNA去甲基化酶,通过调节mRNA修饰在癌症生物学中发挥关键作用。它的解除影响肿瘤细胞的增殖、转移、免疫逃避和治疗抵抗。通过去除m6A甲基化标记,FTO可以改变关键癌基因和肿瘤抑制基因的稳定性和翻译。这些修饰直接影响了参与癌症进展的基本细胞通路,如磷脂酰肌醇3-激酶/蛋白激酶B (PI3K/AKT)、Wnt/β-catenin和哺乳动物雷帕霉素靶蛋白(mTOR)信号通路。本文综述了FTO在肿瘤发病机制中的作用,重点探讨了FTO在免疫调节和化疗反应中的双重作用。在免疫方面,FTO已被证明通过调节免疫检查点的表达和影响肿瘤微环境来促进免疫逃避。此外,FTO对自噬、糖酵解和细胞凋亡抵抗的影响进一步复杂化了化疗的有效性。通过讨论FTO如何调节这些过程的分子细节,我们提供了FTO如何作为一个有希望的治疗靶点来克服癌症相关的挑战,包括免疫抵抗和化疗失败的见解。最后,我们评估了当前和新兴的针对FTO的癌症治疗策略,强调了其增强免疫治疗和化疗结果的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
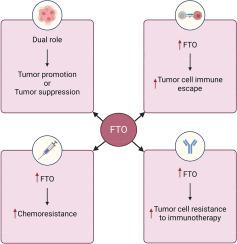
Mechanistic role of FTO in cancer pathogenesis, immune evasion, chemotherapy resistance, and immunotherapy response
Fat mass and obesity-associated protein (FTO), an N6-methyladenosine (m6A) RNA demethylase, plays a key role in cancer biology by regulating mRNA modifications. Its deregulation affects tumor cell proliferation, metastasis, immune evasion, and therapeutic resistance. By removing m6A methylation marks, FTO can alter the stability and translation of key oncogenes and tumor suppressor genes. These modifications directly influence essential cellular pathways involved in cancer progression, such as the phosphatidylinositol 3-kinases/ protein kinase B (PI3K/AKT), Wnt/β-catenin, and mammalian target of rapamycin (mTOR) signaling pathways. This review explores the mechanistic roles of FTO in cancer pathogenesis, focusing on its dual impact on immune regulation and chemotherapy response. In terms of immunity, FTO has been shown to promote immune evasion by modulating the expression of immune checkpoints and influencing the tumor microenvironment. Additionally, FTO’s influence on autophagy, glycolysis, and apoptosis resistance further complicates the effectiveness of chemotherapy treatments. By discussing the molecular details of how FTO regulates these processes, we provide insights into how FTO could serve as a promising therapeutic target to overcome cancer-related challenges, including immune resistance and chemotherapy failure. Finally, we evaluate current and emerging strategies for targeting FTO in cancer therapy, highlighting its potential to enhance immunotherapy and chemotherapy outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Seminars in oncology
医学-肿瘤学
CiteScore
6.60
自引率
0.00%
发文量
58
审稿时长
104 days
期刊介绍:
Seminars in Oncology brings you current, authoritative, and practical reviews of developments in the etiology, diagnosis and management of cancer. Each issue examines topics of clinical importance, with an emphasis on providing both the basic knowledge needed to better understand a topic as well as evidence-based opinions from leaders in the field. Seminars in Oncology also seeks to be a venue for sharing a diversity of opinions including those that might be considered "outside the box". We welcome a healthy and respectful exchange of opinions and urge you to approach us with your insights as well as suggestions of topics that you deem worthy of coverage. By helping the reader understand the basic biology and the therapy of cancer as they learn the nuances from experts, all in a journal that encourages the exchange of ideas we aim to help move the treatment of cancer forward.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


